The polarity of bonds is a fundamental concept in chemistry that describes the distribution of electrical charge across a chemical bond between two atoms. Understanding bond polarity is crucial for predicting the behavior of molecules, their interactions, and their physical properties. This article aims to provide an exhaustive overview of bond polarity, including its definition, the factors that influence it, types of bonds, implications, applications, and illustrative explanations of each concept to enhance understanding.
Definition of Bond Polarity
1. Basic Definition:
- Bond polarity refers to the unequal sharing of electrons between two atoms in a chemical bond, resulting in a dipole moment. A dipole moment occurs when there is a separation of positive and negative charges within the bond, leading to one end of the bond being slightly positive (δ+) and the other end being slightly negative (δ-).
Illustrative Explanation: Imagine a tug-of-war game between two teams (atoms) pulling on a rope (shared electrons). If one team is stronger (more electronegative), they will pull the rope closer to their side, creating an imbalance. This imbalance represents bond polarity, where one atom has a partial negative charge and the other has a partial positive charge.
2. Electronegativity:
- Electronegativity is a measure of an atom’s ability to attract and hold onto electrons in a bond. The difference in electronegativity between two bonded atoms determines the polarity of the bond. The Pauling scale is commonly used to quantify electronegativity, with fluorine being the most electronegative element.
Illustrative Example: Think of electronegativity as a popularity contest among students in a school. The most popular student (fluorine) attracts more friends (electrons) than others. If two students with different popularity levels (electronegativities) form a friendship (bond), the more popular student will have more influence over the relationship, leading to an unequal sharing of friends (electrons).
Factors Influencing Bond Polarity
1. Difference in Electronegativity:
- The greater the difference in electronegativity between two atoms, the more polar the bond. If the difference is significant (typically greater than 1.7), the bond is considered ionic. If the difference is moderate (between 0.4 and 1.7), the bond is polar covalent. If the difference is small (less than 0.4), the bond is nonpolar covalent.
Illustrative Explanation: Imagine two friends deciding how to split a pizza. If one friend (more electronegative) insists on taking a larger slice (more electrons), the other friend (less electronegative) will end up with a smaller piece. The larger the difference in their preferences (electronegativity), the more unequal the pizza distribution (bond polarity).
2. Molecular Geometry:
- The shape of a molecule can influence its overall polarity. Even if a molecule contains polar bonds, the molecular geometry can lead to a nonpolar molecule if the dipoles cancel each other out.
Illustrative Example: Picture a seesaw with two children of equal weight sitting on opposite ends. If they both push down equally (polar bonds), the seesaw remains balanced (nonpolar molecule). However, if one child is heavier (more electronegative), the seesaw tips, creating an imbalance (polar molecule).
3. Presence of Lone Pairs:
- Lone pairs of electrons on the central atom can also affect molecular polarity. Lone pairs can create regions of negative charge, influencing the overall dipole moment of the molecule.
Illustrative Explanation: Imagine a room where a group of friends is gathered. If one friend (lone pair) stands in a corner, they create a shadow (negative charge) that affects how light (dipole moment) is distributed in the room. This shadow can influence how the other friends (atoms) interact with each other.
Types of Bonds Based on Polarity
1. Nonpolar Covalent Bonds:
- In nonpolar covalent bonds, electrons are shared equally between two identical atoms or atoms with similar electronegativities. Examples include diatomic molecules like
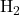 ,
, 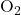 , and
, and 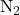 .
.
Illustrative Example: Think of two identical twins sharing a toy equally. They both have the same interest in the toy (electrons), so there is no conflict or imbalance in their sharing (nonpolar bond).
2. Polar Covalent Bonds:
- In polar covalent bonds, electrons are shared unequally between two atoms with different electronegativities. This results in a partial positive charge on one atom and a partial negative charge on the other. An example is the bond in water (
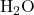 ).
).
Illustrative Explanation: Imagine a couple sharing a blanket. If one partner (more electronegative atom) pulls the blanket closer to themselves, they end up with more coverage (electrons), while the other partner (less electronegative atom) has less. This unequal sharing creates a sense of imbalance (polar bond).
3. Ionic Bonds:
- Ionic bonds occur when the difference in electronegativity between two atoms is so large that one atom completely transfers its electrons to the other, resulting in the formation of charged ions. An example is sodium chloride (
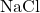 ).
).
Illustrative Example: Picture a game of catch where one player (more electronegative atom) throws the ball (electron) to another player (less electronegative atom). If the throw is strong enough, the ball is completely transferred, and the first player becomes a “thrower” (positive ion), while the second player becomes a “catcher” (negative ion). This complete transfer creates a strong attraction between the two players (ionic bond).
Implications of Bond Polarity
1. Physical Properties:
- The polarity of bonds influences the physical properties of substances, such as boiling points, melting points, and solubility. Polar molecules tend to have higher boiling points than nonpolar molecules due to stronger intermolecular forces (dipole-dipole interactions).
Illustrative Explanation: Imagine two types of water balloons: one filled with water (polar) and the other with oil (nonpolar). When heated, the water balloon (polar) takes longer to burst due to stronger interactions between the water molecules, while the oil balloon (nonpolar) bursts more easily. This illustrates how polarity affects physical properties.
2. Chemical Reactivity:
- The polarity of bonds can affect the reactivity of molecules. Polar molecules are often more reactive in certain types of chemical reactions, such as nucleophilic substitutions, due to the presence of partial charges.
Illustrative Example: Think of a magnet (polar molecule) that attracts small metal objects (reactants). The magnet’s polarity allows it to interact more effectively with the metal, leading to a stronger reaction. In contrast, a nonpolar object would not attract the metal, resulting in less reactivity.
3. Biological Significance:
- In biological systems, the polarity of molecules is crucial for processes such as enzyme-substrate interactions, membrane transport, and the formation of biological structures like proteins and nucleic acids.
Illustrative Explanation: Imagine a key (enzyme) fitting into a lock (substrate). The key’s shape and polarity must match the lock for it to work effectively. If the key is too different (nonpolar), it won’t fit, illustrating how molecular polarity is essential for biological functions.
Applications of Bond Polarity
1. Solubility:
- The principle of “like dissolves like” is based on bond polarity. Polar solvents, such as water, dissolve polar solutes, while nonpolar solvents, such as hexane, dissolve nonpolar solutes. This concept is crucial in chemistry and biochemistry for understanding solubility and reaction mechanisms.
Illustrative Example: Picture a party where guests (solutes) are mingling. Polar guests (polar solutes) feel comfortable in a room filled with other polar guests (polar solvents), while nonpolar guests (nonpolar solutes) prefer a separate room. This illustrates how polarity affects solubility.
2. Material Science:
- Understanding bond polarity is essential in material science for designing new materials with specific properties. For example, polar materials may be used in applications requiring strong adhesive properties, while nonpolar materials may be used for water-repellent coatings.
Illustrative Explanation: Imagine a construction site where workers (materials) are building a structure. If the workers are compatible (similar polarity), they can work together effectively. However, if they are incompatible (different polarity), the structure may not hold together well, illustrating the importance of bond polarity in material design.
3. Pharmaceuticals:
- In drug design, the polarity of molecules is crucial for determining how well a drug will interact with its target. Polar drugs may be more effective in aqueous environments, while nonpolar drugs may be better suited for lipid environments.
Illustrative Example: Think of a delivery truck (drug) that needs to navigate different types of roads (environments). A truck designed for smooth highways (polar) will perform well in urban areas (aqueous environments), while a truck designed for off-road conditions (nonpolar) will excel in rural areas (lipid environments). This illustrates how polarity influences drug effectiveness.
Conclusion
The polarity of bonds is a fundamental concept that underpins our understanding of molecular interactions, chemical reactivity, and the physical properties of substances. By exploring its definition, influencing factors, types of bonds, implications, and applications, we gain valuable insights into how molecules behave and interact in various contexts. Just as a skilled conductor leads an orchestra to create a harmonious performance, the concept of bond polarity orchestrates the interactions of atoms and molecules, allowing us to predict and control their behavior. By mastering this concept, we equip ourselves with the knowledge to analyze, predict, and influence chemical behavior, enhancing our understanding of chemistry and its applications in various fields. Whether in material science, pharmaceuticals, or biological systems, the principles of bond polarity are integral to the functioning of our world and our daily experiences.