Calcium sulphate, a chemical compound with the formula ![]() , is an inorganic salt that plays a significant role in various industrial, agricultural, and biological applications. It exists in several forms, including anhydrous calcium sulphate, dihydrate (gypsum), and hemihydrate (plaster of Paris). This article will delve into the properties, forms, production methods, applications, and significance of calcium sulphate, providing illustrative explanations to enhance understanding.
, is an inorganic salt that plays a significant role in various industrial, agricultural, and biological applications. It exists in several forms, including anhydrous calcium sulphate, dihydrate (gypsum), and hemihydrate (plaster of Paris). This article will delve into the properties, forms, production methods, applications, and significance of calcium sulphate, providing illustrative explanations to enhance understanding.
Chemical Structure and Properties
1. Chemical Formula: The chemical formula for calcium sulphate is ![]() , indicating that it consists of one calcium ion (
, indicating that it consists of one calcium ion (![]() ) and one sulphate ion (
) and one sulphate ion (![]() ). The calcium ion is a cation, while the sulphate ion is an anion, and together they form a neutral compound.
). The calcium ion is a cation, while the sulphate ion is an anion, and together they form a neutral compound.
Illustrative Explanation: Think of calcium sulphate as a balanced team in a sports game. The calcium ion represents a strong player (the cation) who provides stability, while the sulphate ion acts as a strategic player (the anion) who helps in forming a cohesive unit. Together, they create a well-functioning team (the compound).
2. Physical Properties: Calcium sulphate is a white, odorless powder or crystalline solid. It is relatively insoluble in water, with a solubility of about 0.2 g per 100 mL at room temperature. The compound has a melting point of approximately 1450 °C and can decompose at higher temperatures.
Illustrative Explanation: Imagine calcium sulphate as a sturdy building made of bricks. Just as the bricks provide strength and stability to the structure, the physical properties of calcium sulphate contribute to its durability and resistance to dissolution in water.
3. Forms of Calcium Sulphate:
- Anhydrous Calcium Sulphate (
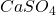 ): This form contains no water molecules and is often used in desiccants and as a drying agent.
): This form contains no water molecules and is often used in desiccants and as a drying agent. - Dihydrate Calcium Sulphate (Gypsum,
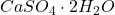 ): This hydrated form contains two water molecules and is widely used in construction, agriculture, and as a food additive.
): This hydrated form contains two water molecules and is widely used in construction, agriculture, and as a food additive. - Hemihydrate Calcium Sulphate (Plaster of Paris,
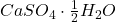 ): This form contains half a water molecule per formula unit and is commonly used in construction and art for making casts and molds.
): This form contains half a water molecule per formula unit and is commonly used in construction and art for making casts and molds.
Illustrative Explanation: Think of the different forms of calcium sulphate as various types of clothing for different occasions. Anhydrous calcium sulphate is like a formal suit (used for specific applications), gypsum is like casual wear (versatile and widely used), and plaster of Paris is like a creative outfit (used for artistic purposes).
Production of Calcium Sulphate
Calcium sulphate can be produced through several methods, including:
1. Natural Sources: Gypsum, the dihydrate form of calcium sulphate, is found naturally in sedimentary rock formations. It can be mined and processed to obtain calcium sulphate.
Illustrative Explanation: Imagine a treasure hunt where miners search for valuable gems. Just as they extract precious stones from the earth, gypsum is mined from natural deposits to obtain calcium sulphate.
2. Chemical Reactions: Calcium sulphate can also be synthesized through chemical reactions. One common method involves the reaction of calcium carbonate (![]() ) with sulfuric acid (
) with sulfuric acid (![]() ):
):
![]()
Illustrative Explanation: Think of this reaction as a cooking recipe. Just as a chef combines ingredients to create a dish, the reaction combines calcium carbonate and sulfuric acid to produce calcium sulphate, carbon dioxide, and water.
3. Hydration and Dehydration: The conversion between different forms of calcium sulphate can occur through hydration and dehydration processes. For example, heating gypsum (dihydrate) at high temperatures can produce plaster of Paris (hemihydrate):
![]()
Illustrative Explanation: Imagine a transformation scene in a movie where a character changes outfits. Just as the character changes from casual wear (gypsum) to a creative outfit (plaster of Paris), the heating process transforms the dihydrate into the hemihydrate form.
Applications of Calcium Sulphate
1. Construction Industry: Calcium sulphate, particularly in the form of gypsum, is widely used in the construction industry for making drywall, plaster, and cement. It provides fire resistance, sound insulation, and thermal properties.
Illustrative Explanation: Think of calcium sulphate as a building block in construction. Just as bricks and mortar create sturdy walls, gypsum and plaster contribute to the structural integrity and safety of buildings.
2. Agriculture: Calcium sulphate is used as a soil amendment to improve soil structure, drainage, and nutrient availability. It helps to reduce soil salinity and provides essential calcium and sulfur nutrients to plants.
Illustrative Explanation: Imagine a gardener nurturing plants in a garden. Just as the gardener enriches the soil with nutrients and improves its structure, calcium sulphate enhances soil quality, promoting healthy plant growth.
3. Food Industry: Calcium sulphate is used as a food additive (E516) in various food products, including tofu, as a coagulant, and in baking powder. It serves as a source of calcium and helps improve texture.
Illustrative Explanation: Think of calcium sulphate as a secret ingredient in a chef’s recipe. Just as the chef adds a special touch to enhance the flavor and texture of a dish, calcium sulphate improves the quality of food products.
4. Medical Applications: Plaster of Paris, a form of calcium sulphate, is commonly used in the medical field for making casts to immobilize broken bones. It hardens quickly and provides support during the healing process.
Illustrative Explanation: Imagine a doctor applying a cast to a patient’s arm. Just as the cast provides stability and support for healing, plaster of Paris serves as a protective layer for injured bones.
5. Desiccants: Anhydrous calcium sulphate is used as a desiccant to absorb moisture in various applications, including packaging, pharmaceuticals, and laboratories.
Illustrative Explanation: Think of anhydrous calcium sulphate as a sponge soaking up water. Just as a sponge absorbs excess moisture to keep things dry, this form of calcium sulphate helps maintain low humidity levels in sensitive environments.
Environmental Considerations
While calcium sulphate has numerous applications, its production and use can have environmental implications. Mining gypsum can lead to habitat disruption, and the chemical processes involved in its synthesis may generate waste products. However, calcium sulphate is generally considered non-toxic and environmentally friendly compared to other chemical compounds.
Illustrative Explanation: Imagine a balance scale weighing benefits against environmental impacts. Just as a responsible individual considers the consequences of their actions, industries must weigh the advantages of using calcium sulphate against its environmental footprint.
Conclusion
Calcium sulphate is a versatile and essential compound with a wide range of applications across various industries, including construction, agriculture, food, and medicine. Its different forms, such as gypsum and plaster of Paris, serve specific purposes, contributing to the quality and functionality of products. Understanding the properties, production methods, and applications of calcium sulphate enhances our appreciation for this important chemical compound. As research continues to explore new uses and sustainable practices, calcium sulphate will remain a vital component in many aspects of modern life.