Thermodynamic processes are fundamental concepts in the field of thermodynamics, which is the branch of physics that deals with heat, work, temperature, and energy transfer. These processes describe how a system changes from one state to another, involving the transfer of energy in the form of heat and work. Understanding thermodynamic processes is crucial for various applications in engineering, chemistry, and physics. This comprehensive overview will explore the definition of thermodynamic processes, types, key concepts, equations, applications, and significance in different contexts.
1. Definition of Thermodynamic Processes
A thermodynamic process is defined as a series of changes that a thermodynamic system undergoes as it moves from one equilibrium state to another. These changes can involve variations in pressure, volume, temperature, and internal energy. The study of thermodynamic processes helps us understand how energy is transferred and transformed within a system and its surroundings.
2. Types of Thermodynamic Processes
Thermodynamic processes can be classified into several types based on specific criteria, such as the nature of heat transfer, the constancy of certain properties, and the direction of energy transfer. The primary types of thermodynamic processes include:
A. Isothermal Process:
An isothermal process occurs at a constant temperature. During this process, any heat added to the system is used to do work, and the internal energy of the system remains constant. For an ideal gas undergoing an isothermal process, the relationship between pressure and volume is described by Boyle’s Law:
![]()
Where:
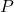 = pressure
= pressure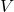 = volume
= volume
B. Adiabatic Process:
An adiabatic process occurs without any heat exchange between the system and its surroundings. In this process, all the work done on or by the system results in a change in internal energy. For an ideal gas undergoing an adiabatic process, the relationship between pressure and volume is described by the equation:
![]()
Where:
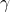 (gamma) = the heat capacity ratio (
(gamma) = the heat capacity ratio (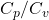 )
)
C. Isochoric Process:
An isochoric process occurs at a constant volume. In this process, no work is done since the volume does not change. Any heat added to the system results in a change in internal energy and temperature. The relationship between heat added (![]() ) and the change in internal energy (
) and the change in internal energy (![]() ) is given by:
) is given by:
![]()
D. Isobaric Process:
An isobaric process occurs at a constant pressure. During this process, the volume of the system can change, and heat added to the system results in both work done and a change in internal energy. The relationship between heat added, work done, and internal energy is given by:
![]()
Where:
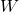 = work done by the system
= work done by the system
3. Key Concepts in Thermodynamic Processes
A. Work (W):
Work is defined as the energy transferred when a force is applied to move an object. In thermodynamics, work can be done by the system (expansion) or on the system (compression). The work done during a process can be calculated using the formula:
![]()
Where:
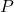 = pressure
= pressure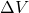 = change in volume
= change in volume
B. Heat (Q):
Heat is the energy transferred between a system and its surroundings due to a temperature difference. Heat transfer can occur through conduction, convection, or radiation. The amount of heat transferred during a process can be calculated using specific heat capacities and temperature changes.
C. Internal Energy (U):
Internal energy is the total energy contained within a system, including kinetic and potential energy at the molecular level. The change in internal energy during a thermodynamic process can be expressed as:
![]()
This equation is a statement of the first law of thermodynamics, which states that energy cannot be created or destroyed, only transformed.
4. Applications of Thermodynamic Processes
Thermodynamic processes have numerous applications across various fields:
A. Heat Engines:
Thermodynamic processes are fundamental to the operation of heat engines, which convert heat energy into mechanical work. Understanding the efficiency and performance of heat engines requires analyzing the thermodynamic processes involved in their operation.
B. Refrigeration and Air Conditioning:
Refrigeration and air conditioning systems rely on thermodynamic processes to transfer heat from a low-temperature reservoir to a high-temperature reservoir. The principles of thermodynamics are essential for designing efficient cooling systems.
C. Chemical Reactions:
In chemistry, thermodynamic processes are crucial for understanding how energy is transferred during chemical reactions. The study of reaction kinetics and thermodynamics helps predict the feasibility and direction of chemical reactions.
D. Material Science:
Thermodynamic processes are important in material science for understanding phase changes, such as melting, boiling, and solidification. These processes are critical for designing materials with specific properties and behaviors.
5. Significance of Thermodynamic Processes
Understanding thermodynamic processes is significant for several reasons:
A. Energy Management:
Thermodynamic processes provide insights into how energy is transferred and transformed, which is essential for managing energy resources efficiently. This understanding is crucial for developing sustainable energy systems.
B. Engineering Design:
In engineering, knowledge of thermodynamic processes is vital for designing systems and devices that involve heat and work, such as engines, turbines, and heat exchangers. Engineers must consider thermodynamic principles to optimize performance and efficiency.
C. Environmental Impact:
Thermodynamic processes play a role in understanding the environmental impact of energy production and consumption. Analyzing these processes helps identify ways to reduce waste and improve energy efficiency, contributing to environmental sustainability.
6. Conclusion
In conclusion, thermodynamic processes are fundamental concepts in thermodynamics that describe how a system changes from one state to another through the transfer of energy in the form of heat and work. The various types of thermodynamic processes, key concepts, applications, and significance highlight their importance in fields such as engineering, chemistry, and environmental science. Understanding these processes is crucial for analyzing energy transfer, designing efficient systems, and addressing global energy challenges. By recognizing the principles of thermodynamic processes, we gain valuable insights into the behavior of energy and matter in the universe.