An acidic buffer solution is a crucial concept in chemistry and biochemistry, playing a vital role in maintaining pH stability in various biological and chemical systems. This article aims to provide a detailed examination of acidic buffer solutions, exploring their definitions, components, mechanisms, applications, and significance in both laboratory and biological contexts.
1. Overview of Acidic Buffer Solutions
1.1 Definition of Acidic Buffer Solution
An acidic buffer solution is a type of buffer that maintains a relatively constant pH when small amounts of acid or base are added. Specifically, an acidic buffer is designed to resist changes in pH in the acidic range, typically around pH 4 to 6. It is composed of a weak acid and its conjugate base, which work together to neutralize added acids or bases, thereby stabilizing the pH of the solution.
1.2 Importance of Acidic Buffer Solutions
Acidic buffer solutions are essential for several reasons:
- pH Regulation: They help maintain the pH of biological fluids, such as blood and cellular environments, which is critical for proper physiological function.
- Chemical Reactions: Many chemical reactions are pH-dependent, and buffers ensure that the conditions remain optimal for these reactions to proceed.
- Laboratory Applications: Acidic buffers are widely used in laboratory settings for various applications, including enzyme assays, titrations, and chromatography.
2. Components of Acidic Buffer Solutions
An acidic buffer solution typically consists of two main components:
2.1 Weak Acid
A weak acid is an acid that does not completely dissociate in solution, meaning that it exists in equilibrium between its undissociated form and its dissociated ions. Common examples of weak acids used in acidic buffers include:
- Acetic Acid (CH₃COOH): A common weak acid that, when dissolved in water, partially dissociates into acetate ions (CH₃COO⁻) and hydrogen ions (H⁺).
- Citric Acid (C₆H₈O₇): A weak organic acid found in citrus fruits, which can act as a buffer in various pH ranges.
- Phosphoric Acid (H₃PO₄): A triprotic acid that can provide buffering capacity at different pH levels, depending on which dissociation step is relevant.
2.2 Conjugate Base
The conjugate base is the species that remains after the weak acid donates a proton (H⁺). It is typically a salt formed from the weak acid. For example:
- Sodium Acetate (CH₃COONa): The conjugate base of acetic acid, which dissociates in solution to provide acetate ions (CH₃COO⁻).
- Potassium Citrate (K₃C₆H₅O₇): The conjugate base of citric acid, which can help maintain pH in acidic environments.
- Sodium Phosphate (Na₃PO₄): The conjugate base of phosphoric acid, which can buffer solutions at various pH levels.
3. Mechanism of Acidic Buffer Solutions
The buffering action of an acidic buffer solution relies on the equilibrium established between the weak acid and its conjugate base. This equilibrium allows the buffer to resist changes in pH when small amounts of strong acids or bases are added. The mechanism can be explained as follows:
3.1 Buffering Action Against Added Acid
When a strong acid (e.g., HCl) is added to the buffer solution, it increases the concentration of hydrogen ions (H⁺) in the solution. The weak acid in the buffer can react with these excess hydrogen ions:
![]()
In this reaction, the weak acid (HA) donates a proton to the added hydrogen ions, forming more of its conjugate base (A⁻) and minimizing the change in pH.
3.2 Buffering Action Against Added Base
Conversely, when a strong base (e.g., NaOH) is added to the buffer solution, it decreases the concentration of hydrogen ions (H⁺) by reacting with them:
![]()
In this case, the conjugate base (A⁻) reacts with the hydroxide ions (OH⁻) from the strong base, forming the weak acid (HA) and water. This reaction helps to neutralize the added base, again minimizing the change in pH.
4. Applications of Acidic Buffer Solutions
Acidic buffer solutions have a wide range of applications in various fields:
4.1 Biological Systems
- Blood pH Regulation: The human body maintains a tightly regulated blood pH of around 7.4, primarily through the bicarbonate buffer system. However, acidic buffers also play a role in specific tissues and cellular environments, ensuring that metabolic processes function optimally.
- Enzyme Activity: Many enzymes require specific pH conditions to function effectively. Acidic buffers help maintain the necessary pH for enzymatic reactions, particularly in metabolic pathways.
4.2 Laboratory Applications
- Biochemical Assays: Acidic buffers are commonly used in biochemical assays to maintain the pH during reactions involving enzymes, proteins, and nucleic acids.
- Titrations: Acidic buffers are employed in titrations to provide a stable pH environment, allowing for accurate determination of the concentration of acids or bases in a solution.
- Chromatography: In techniques such as high-performance liquid chromatography (HPLC), acidic buffers are used to control the pH of mobile phases, affecting the separation of compounds.
4.3 Industrial Applications
- Food Industry: Acidic buffers are used in food preservation and processing to maintain the desired pH for flavor, texture, and safety.
- Pharmaceuticals: Acidic buffers are essential in the formulation of medications, ensuring stability and efficacy by maintaining the appropriate pH.
5. Preparing an Acidic Buffer Solution
The preparation of an acidic buffer solution involves the following steps:
5.1 Selecting Components
Choose a weak acid and its conjugate base that will provide the desired pH range. For example, to prepare a buffer at pH 5, acetic acid and sodium acetate can be selected.
5.2 Calculating Concentrations
Use the Henderson-Hasselbalch equation to determine the appropriate concentrations of the weak acid and conjugate base needed to achieve the desired pH:
![]()
Where:
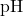 is the desired pH of the buffer.
is the desired pH of the buffer.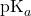 is the negative logarithm of the acid dissociation constant of the weak acid.
is the negative logarithm of the acid dissociation constant of the weak acid.![Rendered by QuickLaTeX.com [\text{A}^-]](https://pengayaan.com/blog/wp-content/uploads/2024/12/quicklatex.com-0703b764075bfa029fca7694b0959c48_l3.png) is the concentration of the conjugate base.
is the concentration of the conjugate base.![Rendered by QuickLaTeX.com [\text{HA}]](https://pengayaan.com/blog/wp-content/uploads/2024/12/quicklatex.com-9785a0d12cb334cbb7e816e10fe5711c_l3.png) is the concentration of the weak acid.
is the concentration of the weak acid.
5.3 Mixing Components
Dissolve the calculated amounts of the weak acid and its conjugate base in a suitable volume of distilled water. Adjust the pH if necessary by adding small amounts of acid or base until the desired pH is achieved.
5.4 Final Adjustments
Once the desired pH is reached, the buffer solution can be diluted to the final volume and stored in a suitable container for future use.
6. Conclusion
In conclusion, acidic buffer solutions are essential tools in both biological and chemical contexts, providing stability and regulation of pH in various systems. Comprised of a weak acid and its conjugate base, these buffers play a critical role in maintaining optimal conditions for enzymatic reactions, metabolic processes, and laboratory applications. Understanding the principles behind acidic buffers, their preparation, and their applications is vital for scientists, researchers, and professionals working in fields ranging from biochemistry to environmental science. As research continues to advance, the importance of acidic buffer solutions in maintaining pH stability and supporting various processes will remain a cornerstone of scientific inquiry and application.