Aliphatic hydrocarbons are a significant class of organic compounds that consist solely of carbon (C) and hydrogen (H) atoms arranged in straight or branched chains, or in non-aromatic rings. Unlike aromatic hydrocarbons, which contain conjugated pi electron systems and exhibit unique stability due to resonance, aliphatic hydrocarbons are characterized by their saturated or unsaturated structures. This article aims to provide a detailed exploration of aliphatic hydrocarbons, including their definitions, classifications, properties, reactions, and applications, along with illustrative explanations of each concept.
Definition of Aliphatic Hydrocarbons
Aliphatic hydrocarbons are organic compounds composed exclusively of carbon and hydrogen atoms, where the carbon atoms are connected in open chains (either straight or branched) or in non-aromatic cyclic structures. The term “aliphatic” is derived from the Greek word “aleiphar,” meaning “fat,” which reflects the fatty nature of many of these compounds. Aliphatic hydrocarbons can be classified into three main categories based on their bonding characteristics: alkanes, alkenes, and alkynes.
Classifications of Aliphatic Hydrocarbons
1. Alkanes (Saturated Hydrocarbons):
- Definition: Alkanes are saturated hydrocarbons that contain only single bonds between carbon atoms. They follow the general formula
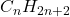 , where
, where 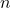 is the number of carbon atoms.
is the number of carbon atoms. - Characteristics: Alkanes are generally non-polar, have low reactivity, and are characterized by their relatively high stability. They are typically found in natural gas and petroleum.
- Illustrative Example: The simplest alkane is methane (
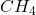 ), which consists of one carbon atom bonded to four hydrogen atoms. As the number of carbon atoms increases, the alkanes become more complex, such as ethane (
), which consists of one carbon atom bonded to four hydrogen atoms. As the number of carbon atoms increases, the alkanes become more complex, such as ethane (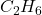 ), propane (
), propane (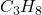 ), and butane (
), and butane (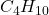 ).
).
2. Alkenes (Unsaturated Hydrocarbons):
- Definition: Alkenes are unsaturated hydrocarbons that contain at least one carbon-carbon double bond. They follow the general formula
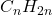 .
. - Characteristics: Alkenes are more reactive than alkanes due to the presence of the double bond, which can participate in various chemical reactions, such as addition reactions. They are commonly used in the production of plastics and other synthetic materials.
- Illustrative Example: Ethylene (
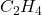 ) is the simplest alkene, consisting of two carbon atoms connected by a double bond. The presence of this double bond allows ethylene to undergo reactions such as polymerization, leading to the formation of polyethylene, a widely used plastic.
) is the simplest alkene, consisting of two carbon atoms connected by a double bond. The presence of this double bond allows ethylene to undergo reactions such as polymerization, leading to the formation of polyethylene, a widely used plastic.
3. Alkynes (Unsaturated Hydrocarbons):
- Definition: Alkynes are unsaturated hydrocarbons that contain at least one carbon-carbon triple bond. They follow the general formula
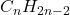 .
. - Characteristics: Alkynes are even more reactive than alkenes due to the presence of the triple bond. They are used in various industrial applications, including the synthesis of pharmaceuticals and as fuel sources.
- Illustrative Example: The simplest alkyne is acetylene (
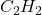 ), which consists of two carbon atoms connected by a triple bond. Acetylene is commonly used as a fuel in welding and cutting applications due to its high flame temperature.
), which consists of two carbon atoms connected by a triple bond. Acetylene is commonly used as a fuel in welding and cutting applications due to its high flame temperature.
Properties of Aliphatic Hydrocarbons
1. Physical Properties:
- State: Aliphatic hydrocarbons can exist in various states at room temperature. Alkanes with fewer than five carbon atoms are typically gases (e.g., methane, ethane), while those with five to about 17 carbon atoms are liquids (e.g., pentane, hexane), and those with more than 17 carbon atoms are usually solids (e.g., paraffin wax).
- Boiling and Melting Points: The boiling and melting points of aliphatic hydrocarbons generally increase with increasing molecular weight due to greater van der Waals forces. For example, the boiling point of methane is -161.5°C, while that of octadecane (
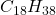 ) is approximately 287°C.
) is approximately 287°C. - Solubility: Aliphatic hydrocarbons are generally non-polar and insoluble in water but soluble in organic solvents such as ether and benzene.
2. Chemical Properties:
- Reactivity: Alkanes are relatively unreactive due to their saturated nature, while alkenes and alkynes are more reactive due to the presence of double and triple bonds, respectively. Alkenes can undergo addition reactions, while alkynes can participate in both addition and substitution reactions.
- Combustion: Aliphatic hydrocarbons readily undergo combustion in the presence of oxygen, producing carbon dioxide and water. For example, the combustion of propane (
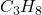 ) can be represented by the equation:
) can be represented by the equation:
![]()
- Substitution Reactions: Alkanes can undergo substitution reactions, particularly with halogens, in the presence of heat or light. For example, the chlorination of methane can be represented as:
![]()
Applications of Aliphatic Hydrocarbons
Aliphatic hydrocarbons have a wide range of applications across various industries:
1. Fuel Sources: Alkanes, particularly those found in natural gas and petroleum, are primary sources of energy. Methane is the main component of natural gas, while gasoline is a mixture of various alkanes used as fuel for internal combustion engines.
2. Chemical Feedstocks: Alkenes and alkynes serve as important feedstocks in the chemical industry. Ethylene is a key precursor for the production of polyethylene, while propylene is used to manufacture polypropylene. Acetylene is utilized in the synthesis of various organic compounds.
3. Solvents: Many aliphatic hydrocarbons, such as hexane and heptane, are used as solvents in laboratories and industrial processes due to their non-polar nature and ability to dissolve a wide range of organic compounds.
4. Lubricants and Greases: Aliphatic hydrocarbons are used in the formulation of lubricants and greases, providing essential properties such as viscosity and stability under varying temperatures.
5. Plastics and Polymers: Alkenes are crucial in the production of various plastics and polymers, which are used in countless applications, from packaging materials to automotive components.
Conclusion
In summary, aliphatic hydrocarbons are a vital class of organic compounds characterized by their carbon and hydrogen composition, which can exist in saturated (alkanes) or unsaturated (alkenes and alkynes) forms. Their diverse properties, reactivity, and wide-ranging applications make them essential in various fields, including energy, materials science, and chemical manufacturing. Through illustrative examples and detailed explanations, we can appreciate the significance of aliphatic hydrocarbons in both theoretical and practical contexts. Understanding these compounds not only enhances our knowledge of organic chemistry but also informs our approach to real-world applications, ensuring the efficient use of these versatile substances in everyday life and industry.