Aromaticity is a fundamental concept in organic chemistry that describes the unique stability and reactivity of certain cyclic compounds. The term “aromatic” originally referred to the pleasant scents of many compounds containing benzene, but it has since evolved to encompass a broader range of chemical properties and behaviors. Understanding aromaticity is crucial for chemists, as it influences the structure, stability, and reactivity of a wide variety of organic molecules. This article will provide a detailed exploration of aromaticity, including its definition, criteria, mechanisms, applications, and illustrative explanations to clarify each concept.
What is Aromaticity?
1. Definition
Aromaticity refers to the property of cyclic compounds that exhibit enhanced stability due to the delocalization of π (pi) electrons across the ring structure. Aromatic compounds typically have a planar structure, a closed loop of p-orbitals, and follow specific rules that distinguish them from non-aromatic and anti-aromatic compounds.
- Illustrative Example: Think of aromaticity as a well-orchestrated symphony. Just as musicians (electrons) come together to create harmonious music (stability), the delocalized π electrons in an aromatic compound work together to enhance the overall stability of the molecule.
2. Historical Context
The concept of aromaticity emerged in the 19th century with the discovery of benzene, a compound that exhibited unusual stability and reactivity compared to other alkenes. Friedrich August Kekulé proposed a cyclic structure for benzene, which laid the groundwork for the modern understanding of aromatic compounds. Over time, the criteria for aromaticity were established, leading to the identification of various aromatic compounds beyond benzene.
- Illustrative Example: Imagine the history of aromaticity as a detective story. The discovery of benzene was the first clue, and chemists like Kekulé pieced together the evidence to uncover the underlying principles of aromaticity, leading to a deeper understanding of organic chemistry.
Criteria for Aromaticity
To determine whether a compound is aromatic, it must meet the following criteria, often referred to as Hückel’s rules:
1. Cyclic Structure
The compound must have a closed ring structure, allowing for the overlap of p-orbitals.
- Illustrative Example: Think of a bicycle wheel as a cyclic structure. Just as the wheel must be closed to function properly, a compound must have a closed ring to exhibit aromaticity.
2. Planarity
The compound must be planar, ensuring that the p-orbitals can overlap effectively. This planarity allows for the delocalization of π electrons across the entire ring.
- Illustrative Example: Imagine a flat piece of paper representing a planar structure. If the paper is crumpled (non-planar), the electrons cannot interact effectively, just as a non-planar compound cannot achieve aromaticity.
3. Conjugated π System
The compound must have a continuous system of p-orbitals, allowing for the delocalization of π electrons. This means that there should be alternating single and double bonds or lone pairs that can participate in π bonding.
- Illustrative Example: Think of a chain of people holding hands, where each person represents a p-orbital. If everyone is connected (conjugated), they can pass a ball (electrons) along the chain, creating a stable system.
4. Hückel’s Rule
The compound must contain a specific number of π electrons, following Hückel’s rule, which states that the number of π electrons must be equal to ![]() , where
, where ![]() is a non-negative integer (0, 1, 2, …). This means that aromatic compounds can have 2, 6, 10, 14, etc., π electrons.
is a non-negative integer (0, 1, 2, …). This means that aromatic compounds can have 2, 6, 10, 14, etc., π electrons.
- Illustrative Example: Imagine a game where players can only win if they have a certain number of points (π electrons). The rule
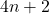 determines the winning scores, ensuring that only specific configurations achieve aromaticity.
determines the winning scores, ensuring that only specific configurations achieve aromaticity.
Types of Aromatic Compounds
Aromatic compounds can be classified into several categories based on their structure and properties:
1. Monocyclic Aromatic Compounds
These compounds contain a single aromatic ring. The most well-known example is benzene (C₆H₆), which has six π electrons and satisfies all the criteria for aromaticity.
- Illustrative Example: Think of a monocyclic aromatic compound as a single-story house. Just as the house has a simple structure, monocyclic compounds have a straightforward arrangement of atoms in one ring.
2. Polycyclic Aromatic Compounds
These compounds consist of multiple fused aromatic rings. Examples include naphthalene (two fused benzene rings) and anthracene (three fused benzene rings). Polycyclic aromatic compounds can exhibit unique properties due to their extended π systems.
- Illustrative Example: Imagine a polycyclic aromatic compound as a multi-story building. Each floor (ring) adds complexity and stability, just as multiple fused rings enhance the properties of the compound.
3. Heteroaromatic Compounds
These compounds contain at least one heteroatom (such as nitrogen, oxygen, or sulfur) in the aromatic ring. Examples include pyridine (a six-membered ring with one nitrogen atom) and furan (a five-membered ring with one oxygen atom). Heteroaromatic compounds can exhibit different reactivity and properties compared to their purely carbon-based counterparts.
- Illustrative Example: Think of heteroaromatic compounds as a mixed salad, where different ingredients (heteroatoms) add unique flavors and textures to the dish (compound), enhancing its overall character.
Mechanism of Aromaticity
The mechanism of aromaticity involves the delocalization of π electrons across the aromatic ring, leading to increased stability. This delocalization can be understood through the following concepts:
1. Resonance
Aromatic compounds can be represented by multiple resonance structures, which illustrate the delocalization of π electrons. The actual structure is a hybrid of these resonance forms, leading to a more stable configuration.
- Illustrative Example: Imagine a group of friends (resonance structures) who can change their outfits (electron arrangements) while still maintaining their core identity (the aromatic compound). The combination of different outfits creates a unique and stable appearance.
2. Stability
The delocalization of π electrons in aromatic compounds results in lower energy and increased stability compared to non-aromatic or anti-aromatic compounds. This stability is often referred to as “aromatic stabilization.”
- Illustrative Example: Think of aromatic stability as a well-built bridge. Just as a sturdy bridge can support heavy loads (stability), the delocalized electrons in an aromatic compound provide a strong foundation that enhances its overall stability.
Applications of Aromatic Compounds
Aromatic compounds have a wide range of applications across various fields, including:
1. Pharmaceuticals
Many drugs contain aromatic rings, which contribute to their biological activity. The unique properties of aromatic compounds allow for specific interactions with biological targets.
- Illustrative Example: Think of aromatic compounds in pharmaceuticals as keys that fit into specific locks (biological targets). The unique shape and properties of the keys (aromatic rings) enable them to unlock the desired effects in the body.
2. Dyes and Pigments
Aromatic compounds are often used in the production of dyes and pigments due to their vibrant colors and stability. The delocalized electrons in aromatic systems contribute to their ability to absorb light.
- Illustrative Example: Imagine aromatic dyes as artists painting a canvas. The rich colors (light absorption) created by the aromatic compounds bring life to the artwork (materials).
3. Plastics and Polymers
Many synthetic polymers contain aromatic groups, which enhance their mechanical properties and thermal stability. Aromatic compounds are essential in the production of materials like polystyrene and polycarbonate.
- Illustrative Example: Think of aromatic compounds in plastics as the steel reinforcements in a concrete structure. Just as steel adds strength and durability to the building, aromatic groups enhance the properties of synthetic materials.
4. Fragrances and Flavorings
Aromatic compounds are widely used in the fragrance and flavor industry due to their pleasant scents and tastes. Many natural and synthetic aromatic compounds contribute to the sensory experience of products.
- Illustrative Example: Imagine aromatic compounds in fragrances as the spices in a gourmet dish. Just as spices enhance the flavor and aroma of food, aromatic compounds add delightful scents to perfumes and flavorings.
Advantages of Aromatic Compounds
Aromatic compounds offer several advantages that contribute to their widespread use:
1. Stability: The delocalization of π electrons provides aromatic compounds with enhanced stability, making them less reactive than non-aromatic compounds.
2. Diverse Applications: Aromatic compounds are versatile and find applications in pharmaceuticals, dyes, plastics, and fragrances, among other fields.
3. Unique Properties: The unique electronic structure of aromatic compounds leads to distinct physical and chemical properties, allowing for tailored applications.
- Illustrative Example: Think of aromatic compounds as multi-talented performers in a theater. Just as a performer can take on various roles (applications) while maintaining their unique style (properties), aromatic compounds excel in diverse fields.
Disadvantages of Aromatic Compounds
Despite their advantages, aromatic compounds also have some limitations:
1. Toxicity: Some aromatic compounds, such as benzene, are toxic and pose health risks. Prolonged exposure can lead to serious health issues, including cancer.
2. Environmental Concerns: The production and disposal of aromatic compounds can lead to environmental pollution, necessitating careful management and regulation.
3. Reactivity: While aromatic compounds are generally stable, certain reactions (such as electrophilic aromatic substitution) can lead to unwanted byproducts if not controlled properly.
- Illustrative Example: Think of aromatic compounds as beautiful flowers in a garden. While they can bring joy and beauty (applications), some flowers (toxic compounds) can also be harmful if not handled with care.
Conclusion
Aromaticity is a fundamental concept in organic chemistry that describes the unique stability and reactivity of cyclic compounds with delocalized π electrons. Understanding the criteria for aromaticity, the types of aromatic compounds, and their mechanisms is essential for anyone studying or working in the field of chemistry. Aromatic compounds play a vital role in various applications, from pharmaceuticals and dyes to plastics and fragrances, showcasing their versatility and importance in modern science and industry. As research continues to advance, the exploration of aromatic compounds will likely lead to new discoveries and innovations, further enhancing their significance in chemistry and beyond. Whether in the laboratory, the manufacturing plant, or the world of nature, aromatic compounds exemplify the beauty and complexity of chemical interactions, highlighting the intricate dance of electrons that underpins the science of chemistry.