Boyle’s Law is one of the fundamental principles in the field of gas physics, named after the Irish scientist Robert Boyle, who first formulated the law in the 17th century. This law describes the relationship between the pressure and volume of a gas at constant temperature, providing critical insights into the behavior of gases under varying conditions. This article aims to provide a detailed understanding of Boyle’s Law, including its definition, mathematical formulation, underlying principles, applications, and illustrative examples.
Definition of Boyle’s Law
Boyle’s Law states that the pressure of a given mass of gas is inversely proportional to its volume when the temperature is held constant. In simpler terms, if the volume of a gas decreases, its pressure increases, and vice versa, provided that the temperature remains unchanged. This relationship can be mathematically expressed as:
![]()
or, more commonly, in the form of the equation:
![]()
where:
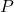 is the pressure of the gas,
is the pressure of the gas,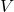 is the volume of the gas,
is the volume of the gas,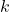 is a constant for a given amount of gas at a constant temperature.
is a constant for a given amount of gas at a constant temperature.
Understanding the Concepts
1. Pressure (P): Pressure is defined as the force exerted per unit area. In the context of gases, it is the result of gas molecules colliding with the walls of their container. Pressure is typically measured in units such as atmospheres (atm), pascals (Pa), or millimeters of mercury (mmHg).
2. Volume (V): Volume refers to the amount of space that a gas occupies. It is usually measured in liters (L) or cubic meters (m³). The volume of a gas can change significantly with variations in pressure and temperature.
3. Constant Temperature: Boyle’s Law applies under isothermal conditions, meaning that the temperature of the gas does not change during the process. This is crucial because temperature affects the kinetic energy of gas molecules, which in turn influences pressure and volume.
Mathematical Formulation
To illustrate Boyle’s Law mathematically, consider a gas contained in a cylinder with a movable piston. If the initial pressure and volume of the gas are ![]() and
and ![]() , and the final pressure and volume after compression or expansion are
, and the final pressure and volume after compression or expansion are ![]() and
and ![]() , Boyle’s Law can be expressed as:
, Boyle’s Law can be expressed as:
![]()
This equation indicates that the product of pressure and volume remains constant for a given amount of gas at a constant temperature.
Graphical Representation
A graphical representation of Boyle’s Law can be quite illuminating. If we plot pressure (P) on the y-axis and volume (V) on the x-axis, we will observe a hyperbolic curve. This curve illustrates the inverse relationship between pressure and volume: as the volume increases, the pressure decreases, and vice versa.
- Isotherm Curves: Each curve on this graph represents a different temperature. As the temperature increases, the curves shift upward, indicating that at higher temperatures, for the same volume, the pressure of the gas is higher.
Applications of Boyle’s Law
Boyle’s Law has numerous practical applications across various fields, including:
1. Respiratory Physiology: In human physiology, Boyle’s Law explains how breathing works. When the diaphragm contracts, the volume of the thoracic cavity increases, leading to a decrease in pressure within the lungs. This pressure drop allows air to flow into the lungs from the atmosphere, demonstrating the principles of gas behavior in biological systems.
Illustrative Example: During inhalation, the diaphragm moves downward, increasing the volume of the thoracic cavity. According to Boyle’s Law, as the volume increases, the pressure inside the lungs decreases. This pressure difference causes air to rush into the lungs, allowing for gas exchange.
2. Syringes and Medical Devices: Boyle’s Law is fundamental in the operation of syringes. When the plunger of a syringe is pulled back, the volume inside the syringe increases, causing the pressure to drop. This pressure drop allows liquid to be drawn into the syringe.
Illustrative Example: When a healthcare professional pulls back on the plunger of a syringe, the volume inside the barrel increases. As a result, the pressure decreases, and the liquid (such as medication) is drawn into the syringe due to the higher external atmospheric pressure.
3. Diving and Scuba: Boyle’s Law is critical for divers, as it explains how pressure changes with depth. As a diver descends, the pressure increases, causing the volume of air in their lungs to decrease. Divers must be aware of this principle to avoid lung over-expansion injuries during ascent.
Illustrative Example: A diver at a depth of 10 meters experiences approximately double the atmospheric pressure compared to the surface. If the diver holds their breath while ascending, the volume of air in their lungs will expand due to the decrease in pressure. If the diver ascends too quickly, this expansion can lead to serious injury, such as a ruptured lung.
4. Gas Storage and Transportation: Boyle’s Law is essential in the design and operation of gas storage tanks and transportation systems. Understanding how gases behave under pressure allows engineers to design safe and efficient systems for storing and transporting gases like natural gas and propane.
Illustrative Example: In a gas cylinder, the gas is stored at high pressure. If the volume of the gas is reduced (for example, by using a smaller cylinder), the pressure will increase. Engineers must account for these changes to ensure that the cylinder can safely contain the gas without risk of explosion.
Limitations of Boyle’s Law
While Boyle’s Law is a powerful tool for understanding gas behavior, it has limitations:
1. Ideal Gas Assumption: Boyle’s Law assumes that gases behave ideally, meaning that the gas molecules do not interact with each other and occupy no volume. In reality, gases can deviate from ideal behavior, especially at high pressures and low temperatures.
2. Non-Ideal Conditions: Under conditions of high pressure or low temperature, real gases may exhibit behaviors that do not conform to Boyle’s Law. In such cases, corrections must be made using the van der Waals equation or other models that account for intermolecular forces and molecular volume.
3. Limited to Isothermal Processes: Boyle’s Law only applies when the temperature is constant. If the temperature changes during the process, the relationship between pressure and volume will also change, necessitating the use of other gas laws, such as Charles’s Law or the Ideal Gas Law.
Conclusion
In conclusion, Boyle’s Law is a fundamental principle that describes the inverse relationship between the pressure and volume of a gas at constant temperature. Its mathematical formulation, graphical representation, and numerous applications in fields such as physiology, medicine, and engineering highlight its significance in understanding gas behavior. While it has limitations, Boyle’s Law remains a cornerstone of gas physics, providing essential insights into the behavior of gases in various contexts. Through illustrative examples and detailed explanations, we can appreciate the profound impact of Boyle’s Law on both theoretical and practical aspects of science and engineering. Understanding this law not only enhances our knowledge of gas behavior but also informs our approach to real-world applications, ensuring safety and efficiency in various systems involving gases.