Chemical formulas are symbolic representations of the composition of chemical compounds. They provide essential information about the types and numbers of atoms present in a substance, as well as the ratios in which these atoms combine. Understanding chemical formulas is fundamental to the study of chemistry, as they are used to convey information about molecular structure, stoichiometry, and chemical reactions. This comprehensive overview will explore the types of chemical formulas, their components, significance, examples, and applications.
1. Types of Chemical Formulas
Chemical formulas can be categorized into several types, each serving a different purpose in conveying information about a compound:
A. Empirical Formula:
- The empirical formula represents the simplest whole-number ratio of the elements in a compound. It does not provide information about the actual number of atoms or the structure of the molecule.
- Example: The empirical formula for glucose (C₆H₁₂O₆) is CH₂O, indicating that the ratio of carbon to hydrogen to oxygen is 1:2:1.
B. Molecular Formula:
- The molecular formula indicates the actual number of atoms of each element in a molecule of a compound. It provides more specific information than the empirical formula.
- Example: The molecular formula for glucose is C₆H₁₂O₆, showing that each molecule contains six carbon atoms, twelve hydrogen atoms, and six oxygen atoms.
C. Structural Formula:
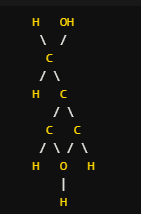
- The structural formula provides a visual representation of the arrangement of atoms within a molecule, including the bonds between them. It can be drawn in various ways, such as Lewis structures, condensed structural formulas, or skeletal formulas.
- Example: The structural formula for glucose can be represented as:
D. Condensed Formula:
- The condensed formula is a shorthand representation that shows the arrangement of atoms in a molecule without depicting all the bonds explicitly. It is often used for larger molecules.
- Example: The condensed formula for butane (C₄H₁₀) can be written as CH₃(CH₂)₂CH₃, indicating the branching structure of the molecule.
2. Components of Chemical Formulas
Chemical formulas consist of several key components that convey important information about the compound:
A. Element Symbols:
- Each element in a chemical formula is represented by its chemical symbol, which is typically one or two letters derived from its name. The first letter is always capitalized, while the second letter, if present, is lowercase.
- Example: In the formula H₂O, “H” represents hydrogen, and “O” represents oxygen.
B. Subscripts:
- Subscripts are numbers written after an element symbol that indicate the number of atoms of that element in the molecule. If no subscript is present, it is understood that there is one atom of that element.
- Example: In CO₂, the subscript “2” indicates that there are two oxygen atoms for every one carbon atom.
C. Parentheses:
- Parentheses are used in chemical formulas to indicate groups of atoms that are repeated in a compound. This is particularly useful for polyatomic ions or complex structures.
- Example: In calcium nitrate, Ca(NO₃)₂, the parentheses indicate that the nitrate ion (NO₃) is present in a quantity of two.
D. Charges:
- In ionic compounds, the charge of ions may be indicated in the formula. Positive charges are represented by superscripts, and negative charges are indicated with a minus sign.
- Example: In sodium chloride (NaCl), sodium has a charge of +1, and chloride has a charge of -1, but these charges are not explicitly shown in the formula since the compound is neutral.
3. Significance of Chemical Formulas
A. Communication:
- Chemical formulas serve as a universal language in chemistry, allowing scientists to communicate the composition and structure of compounds clearly and concisely.
B. Stoichiometry:
- Chemical formulas are essential for stoichiometric calculations, which involve determining the quantities of reactants and products in chemical reactions. They provide the necessary information to balance chemical equations and predict the outcomes of reactions.
C. Understanding Properties:
- The chemical formula of a compound can provide insights into its physical and chemical properties. For example, the presence of functional groups in organic compounds can influence their reactivity and behavior.
D. Predicting Reactions:
- Knowledge of chemical formulas allows chemists to predict how different substances will react with one another, facilitating the design of experiments and the development of new materials.
4. Examples of Chemical Formulas
A. Simple Compounds:
- Water (H₂O): A compound consisting of two hydrogen atoms and one oxygen atom.
- Sodium Chloride (NaCl): An ionic compound formed from sodium and chloride ions.
B. Organic Compounds:
- Ethanol (C₂H₅OH): A simple alcohol with two carbon atoms, six hydrogen atoms, and one hydroxyl group.
- Acetic Acid (C₂H₄O₂): An organic compound with two carbon atoms, four hydrogen atoms, and two oxygen atoms, commonly known as vinegar.
C. Complex Compounds:
- Glucose (C₆H₁₂O₆): A simple sugar with six carbon atoms, twelve hydrogen atoms, and six oxygen atoms, essential for energy in living organisms.
- Aspirin (C₉H₈O₄): A widely used pharmaceutical compound with nine carbon atoms, eight hydrogen atoms, and four oxygen atoms.
5. Applications of Chemical Formulas
A. Pharmaceutical Chemistry:
- Chemical formulas are crucial in the development and formulation of drugs, allowing chemists to understand the structure and function of active ingredients.
B. Environmental Science:
- In environmental chemistry, chemical formulas are used to analyze pollutants and understand their behavior in ecosystems, aiding in the development of remediation strategies.
C. Material Science:
- Chemical formulas are essential in the design and synthesis of new materials, including polymers, ceramics, and nanomaterials, by providing insights into their composition and properties.
D. Education:
- Chemical formulas are fundamental in teaching chemistry, helping students learn about the composition of substances, chemical reactions, and the principles of stoichiometry.
6. Conclusion
In conclusion, chemical formulas are vital tools in the field of chemistry that provide essential information about the composition, structure, and properties of substances. By understanding the different types of chemical formulas, their components, and their significance, one can gain valuable insights into the behavior of matter and the principles governing chemical reactions. As research and technology continue to advance, the role of chemical formulas will remain central to the exploration of new compounds, materials, and processes, ultimately contributing to our understanding of the natural world and the development of innovative solutions to complex challenges.