Conduction is one of the three primary modes of heat transfer, alongside convection and radiation. It is the process by which heat energy is transferred through a material without any movement of the material itself. This article will provide an in-depth examination of conduction, covering its definition, mechanisms, types, mathematical descriptions, examples, and applications, along with illustrative explanations to enhance understanding.
1. Definition of Conduction
Conduction is defined as the transfer of thermal energy through a material due to a temperature difference, where heat flows from the hotter region to the cooler region. This process occurs at the microscopic level as particles collide and transfer energy to neighboring particles. Conduction is most effective in solids, particularly metals, due to their closely packed particles and free electrons that facilitate energy transfer.
Illustrative Explanation: Imagine a metal spoon placed in a hot cup of coffee. The end of the spoon in contact with the coffee heats up first. As the molecules at that end gain energy, they vibrate more vigorously and collide with adjacent molecules, transferring energy along the length of the spoon. Eventually, the heat travels to the other end of the spoon, which may be cool to the touch initially, but becomes warm as energy is conducted through the material.
2. Mechanisms of Conduction
The conduction process can be understood through two primary mechanisms: lattice vibrations and free electron movement.
- Lattice Vibrations: In solids, atoms are arranged in a fixed lattice structure. When one part of the lattice is heated, the atoms in that region vibrate more vigorously. These vibrations are transmitted to neighboring atoms through collisions, allowing energy to flow through the material. This mechanism is particularly significant in non-metallic solids, such as ceramics and insulators.
- Free Electron Movement: In metals, conduction is enhanced by the presence of free electrons. Metals have a structure that allows some electrons to move freely throughout the lattice. When a metal is heated, these free electrons gain kinetic energy and move rapidly, colliding with other electrons and atoms, thereby transferring energy efficiently. This is why metals are excellent conductors of heat.
Illustrative Explanation: Consider a metal rod heated at one end. The free electrons at the heated end gain energy and move faster. As they collide with other electrons and atoms, they transfer energy, causing the entire rod to heat up. In contrast, if you heat a non-metallic material, the energy transfer relies solely on the vibrations of the atoms, which is less efficient.
3. Types of Conduction
Conduction can be categorized into two main types based on the materials involved:
- Thermal Conduction: This is the most common form of conduction, referring to the transfer of heat through a material. It occurs in solids, liquids, and gases, but is most efficient in solids due to their closely packed particles.
- Electrical Conduction: This type of conduction refers to the transfer of electric charge through a material. It is particularly relevant in conductive materials like metals, where free electrons facilitate the flow of electric current. The principles of electrical conduction are closely related to thermal conduction, as both involve the movement of charged particles.
Illustrative Explanation: To illustrate thermal conduction, think of a metal frying pan on a hot stove. The heat from the stove is conducted through the metal to the food inside the pan. For electrical conduction, consider a copper wire connected to a battery. When the circuit is completed, free electrons in the copper wire move toward the positive terminal of the battery, allowing electric current to flow.
4. Mathematical Description of Conduction
The rate of heat transfer through conduction can be quantified using Fourier’s Law of Heat Conduction, which states that the heat transfer rate (Q) through a material is proportional to the temperature gradient (ΔT) and the area (A) through which heat is being transferred, and inversely proportional to the thickness (d) of the material. The mathematical expression is given by:
![]()
Where:
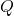 is the heat transfer rate (in watts, W).
is the heat transfer rate (in watts, W).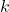 is the thermal conductivity of the material (in watts per meter per degree Celsius, W/m·°C).
is the thermal conductivity of the material (in watts per meter per degree Celsius, W/m·°C).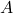 is the cross-sectional area through which heat is being conducted (in square meters, m²).
is the cross-sectional area through which heat is being conducted (in square meters, m²).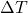 is the temperature difference across the material (in degrees Celsius, °C).
is the temperature difference across the material (in degrees Celsius, °C).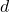 is the thickness of the material (in meters, m).
is the thickness of the material (in meters, m).
Illustrative Explanation: Imagine a wall separating two rooms, one heated and the other cold. The rate at which heat flows through the wall depends on the wall’s thickness, the area of the wall, the temperature difference between the rooms, and the material’s thermal conductivity. A thicker wall or a material with low thermal conductivity will reduce the heat transfer rate, while a larger temperature difference will increase it.
5. Examples of Conduction
Conduction is a common phenomenon encountered in everyday life. Here are a few illustrative examples:
- Cooking: When cooking on a stovetop, heat is conducted from the burner to the pot and then to the food. The efficiency of heat transfer depends on the material of the pot; for instance, copper pots conduct heat better than stainless steel pots.
- Heating Systems: In a radiator heating system, hot water flows through pipes, and heat is conducted from the water to the metal radiator, which then warms the surrounding air.
- Insulation: Insulating materials, such as fiberglass or foam, are designed to reduce heat conduction. They have low thermal conductivity, which minimizes heat loss in buildings, keeping them warm in winter and cool in summer.
Illustrative Explanation: Consider a metal baking sheet placed in an oven. The heat from the oven air is conducted through the metal to the food on the sheet. If you were to use a glass or ceramic dish instead, the heat transfer would be less efficient, resulting in longer cooking times.
6. Applications of Conduction
Conduction has numerous practical applications across various fields:
- Engineering: Understanding conduction is crucial in designing thermal systems, such as heat exchangers, where efficient heat transfer is necessary for energy conservation.
- Electronics: In electronic devices, managing heat conduction is vital to prevent overheating. Heat sinks are often used to dissipate heat away from components, ensuring they operate within safe temperature limits.
- Material Science: The study of thermal conductivity is essential in developing new materials for specific applications, such as insulators for buildings or conductors for electrical wiring.
- Thermal Management: In industries such as aerospace and automotive, effective thermal management systems are designed to control heat conduction, ensuring optimal performance and safety.
Illustrative Explanation: In a computer, the CPU generates heat during operation. To prevent overheating, a heat sink made of a highly conductive material, like aluminum or copper, is attached to the CPU. The heat is conducted away from the CPU to the heat sink, where it can be dissipated into the surrounding air, keeping the CPU cool and functioning efficiently.
Conclusion
Conduction is a fundamental process that plays a critical role in heat transfer and energy management across various applications. By understanding the mechanisms of conduction, its types, mathematical descriptions, and real-world examples, we can appreciate its significance in both natural phenomena and technological advancements. From cooking to engineering, conduction is an essential concept that underpins many aspects of our daily lives and the functioning of the world around us. As we continue to explore and innovate, a deeper understanding of conduction will enable us to develop more efficient systems and materials, ultimately enhancing our quality of life and advancing scientific knowledge.