Critical temperature is a fundamental concept in thermodynamics and physical chemistry that refers to the highest temperature at which a substance can exist as a liquid, regardless of the pressure applied. Above this temperature, the substance cannot be liquefied by pressure alone, and it exists solely in the gaseous state. Understanding critical temperature is essential for various scientific and industrial applications, including the study of phase transitions, the behavior of gases and liquids, and the design of refrigeration systems. In this comprehensive exploration of critical temperature, we will delve into its definition, significance, factors influencing it, applications, and its role in various fields of science and engineering.
Definition of Critical Temperature
Critical temperature (![]() ) is defined as the temperature above which a substance cannot exist in the liquid phase, regardless of the pressure applied. At this temperature, the properties of the liquid and gas phases become indistinguishable, leading to the formation of a supercritical fluid. A supercritical fluid exhibits unique properties that are intermediate between those of gases and liquids, making it an important subject of study in various scientific fields.
) is defined as the temperature above which a substance cannot exist in the liquid phase, regardless of the pressure applied. At this temperature, the properties of the liquid and gas phases become indistinguishable, leading to the formation of a supercritical fluid. A supercritical fluid exhibits unique properties that are intermediate between those of gases and liquids, making it an important subject of study in various scientific fields.
Phase Diagram and Critical Point
To understand critical temperature, it is essential to consider the phase diagram of a substance. A phase diagram is a graphical representation that shows the equilibrium states of a substance as a function of temperature and pressure. The key features of a phase diagram include:
1. Phases: The diagram typically includes regions representing the solid, liquid, and gas phases of the substance.
2. Phase Boundaries: The lines separating the different phases indicate the conditions under which phase transitions occur. For example, the line between the liquid and gas phases represents the boiling point at various pressures.
3. Critical Point: The critical point is the specific point on the phase diagram where the liquid-gas boundary terminates. At this point, the critical temperature and critical pressure (![]() ) are defined. Beyond the critical point, the distinction between liquid and gas phases disappears, and the substance exists as a supercritical fluid.
) are defined. Beyond the critical point, the distinction between liquid and gas phases disappears, and the substance exists as a supercritical fluid.
Mathematical Representation
The relationship between critical temperature, critical pressure, and critical volume can be described using the van der Waals equation, which accounts for the non-ideal behavior of real gases. The van der Waals equation is given by:
![]()
Where:
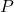 is the pressure of the gas.
is the pressure of the gas.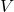 is the volume of the gas.
is the volume of the gas.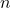 is the number of moles of the gas.
is the number of moles of the gas.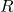 is the universal gas constant.
is the universal gas constant.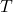 is the temperature.
is the temperature. and
and 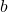 are constants specific to the gas, accounting for intermolecular forces and molecular volume, respectively.
are constants specific to the gas, accounting for intermolecular forces and molecular volume, respectively.
At the critical point, the first and second derivatives of pressure with respect to volume become equal to zero, leading to the critical conditions defined by:
![]()
![]()
![]()
These equations illustrate the interdependence of critical temperature, critical pressure, and critical volume for a given substance.
Factors Influencing Critical Temperature
Several factors influence the critical temperature of a substance:
- Intermolecular Forces: The strength of intermolecular forces, such as hydrogen bonding, van der Waals forces, and dipole-dipole interactions, significantly affects critical temperature. Substances with stronger intermolecular forces tend to have higher critical temperatures because more energy is required to overcome these forces and transition to the gaseous state.
- Molecular Structure: The molecular structure and size of a substance also play a crucial role in determining its critical temperature. Larger molecules with more complex structures may exhibit higher critical temperatures due to increased van der Waals forces.
- Chemical Composition: The chemical composition of a substance can influence its critical temperature. For example, polar molecules generally have higher critical temperatures than nonpolar molecules due to stronger dipole-dipole interactions.
- Pressure: While critical temperature is defined as the temperature above which a substance cannot be liquefied by pressure alone, the critical pressure itself can influence the critical temperature. For example, increasing the pressure can raise the boiling point of a liquid, but it does not change the critical temperature.
Applications of Critical Temperature
Critical temperature has a wide range of applications across various fields, including:
- Supercritical Fluids: Supercritical fluids, which exist above the critical temperature and pressure, have unique properties that make them useful in various applications. For example, supercritical carbon dioxide is used as a solvent in extraction processes, such as decaffeination of coffee and extraction of essential oils.
- Refrigeration and Cryogenics: Understanding critical temperature is essential in the design of refrigeration systems and cryogenic applications. Refrigerants must be selected based on their critical temperatures to ensure efficient heat transfer and phase transitions.
- Chemical Engineering: In chemical engineering, critical temperature is important for designing reactors and separation processes. Knowledge of the critical temperature helps engineers optimize conditions for reactions and separations involving gases and liquids.
- Material Science: Critical temperature plays a role in the study of phase transitions in materials. Understanding the critical temperature of polymers, metals, and other materials is crucial for predicting their behavior under different thermal conditions.
- Environmental Science: In environmental science, critical temperature is relevant for understanding the behavior of gases in the atmosphere. For example, the critical temperature of greenhouse gases can influence their behavior and interactions in the atmosphere.
Significance of Critical Temperature
The significance of critical temperature extends beyond its definition and applications. Some key points of significance include:
- Understanding Phase Transitions: Critical temperature is a key parameter in understanding phase transitions, which are fundamental to many physical and chemical processes. Knowledge of critical temperature helps scientists and engineers predict how substances will behave under varying conditions.
- Predictive Power: The concept of critical temperature allows for the prediction of the behavior of substances in different states. This predictive power is essential for designing processes and systems in various scientific and industrial applications.
- Foundation for Advanced Theories: Critical temperature serves as a foundation for more advanced theories in thermodynamics and statistical mechanics. It is a critical parameter in the study of critical phenomena, such as phase transitions and critical behavior in systems.
- Educational Value: The concept of critical temperature is an essential part of the chemistry and physics curriculum. It provides students with a deeper understanding of thermodynamics, phase behavior, and the properties of materials.
Conclusion
In conclusion, critical temperature is a fundamental concept in thermodynamics and physical chemistry that defines the highest temperature at which a substance can exist as a liquid, regardless of pressure. Its definition, significance, factors influencing it, applications, and role in various fields highlight its importance in understanding phase transitions and the behavior of substances. As research and technology continue to evolve, the study of critical temperature will remain a vital area of exploration, driving advancements in science, engineering, and materials development. Understanding critical temperature not only enhances our knowledge of thermodynamic principles but also informs practical applications that impact our daily lives and the advancement of technology.