Esters are a class of organic compounds formed through the reaction of an alcohol and a carboxylic acid. They are characterized by their distinctive functional group, which is represented by the formula ![]() , where
, where ![]() and
and ![]() are hydrocarbon chains or groups. Esters are widely known for their pleasant fragrances and flavors, making them important in various industries, including food, cosmetics, and pharmaceuticals. This article will provide an exhaustive exploration of esters, covering their chemical structure, properties, formation, types, applications, and significance in different contexts.
are hydrocarbon chains or groups. Esters are widely known for their pleasant fragrances and flavors, making them important in various industries, including food, cosmetics, and pharmaceuticals. This article will provide an exhaustive exploration of esters, covering their chemical structure, properties, formation, types, applications, and significance in different contexts.
Chemical Structure of Esters
1. Functional Group: The defining feature of esters is the ester functional group, which consists of a carbonyl group (![]() ) adjacent to an ether group (
) adjacent to an ether group (![]() ). The general structure of an ester can be represented as:
). The general structure of an ester can be represented as:
![]()
In this structure:
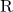 represents the alkyl group derived from the carboxylic acid.
represents the alkyl group derived from the carboxylic acid.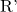 represents the alkyl group derived from the alcohol.
represents the alkyl group derived from the alcohol.
2. Nomenclature: Esters are named by combining the name of the alcohol and the name of the carboxylic acid from which they are derived. The alkyl group from the alcohol is named first, followed by the name of the acid with the suffix “-oate” replacing “-ic acid.” For example, the ester formed from ethanol and acetic acid is called ethyl acetate.
3. Example Structure: Consider the formation of ethyl acetate from acetic acid and ethanol:
- Acetic Acid:
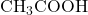
- Ethanol:
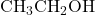
- Ethyl Acetate:
![]()
Properties of Esters
Esters possess several unique physical and chemical properties that distinguish them from other organic compounds:
1. Physical Properties:
- State: Many esters are liquids at room temperature, although some can be solids or gases.
- Odor: Esters are often characterized by their pleasant, fruity odors, which is why they are commonly used in flavorings and fragrances.
- Solubility: Lower molecular weight esters (up to about 4-5 carbon atoms) are generally soluble in water due to their ability to form hydrogen bonds with water molecules. However, as the carbon chain length increases, their solubility in water decreases, while their solubility in organic solvents increases.
- Boiling Point: Esters typically have lower boiling points than their corresponding carboxylic acids due to the absence of hydrogen bonding between ester molecules.
2. Chemical Properties:
- Reactivity: Esters can undergo hydrolysis, a reaction with water that breaks them down into their constituent alcohol and carboxylic acid. This reaction can be catalyzed by acids or bases.
- Transesterification: Esters can react with alcohols to form new esters and alcohols in a process known as transesterification. This reaction is important in biodiesel production.
- Saponification: The reaction of esters with strong bases (such as sodium hydroxide) leads to the formation of soap and glycerol, a process known as saponification.
Formation of Esters
Esters are primarily formed through a reaction known as esterification, which involves the condensation of an alcohol and a carboxylic acid. The general reaction can be represented as follows:
![]()
In this reaction:
- The hydroxyl group (
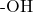 ) from the carboxylic acid and a hydrogen atom from the alcohol combine to form water (
) from the carboxylic acid and a hydrogen atom from the alcohol combine to form water (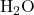 ).
). - The remaining parts of the acid and alcohol combine to form the ester.
Catalysis: The reaction is typically catalyzed by an acid (such as sulfuric acid) to increase the rate of ester formation. The presence of heat can also drive the reaction forward.
Reversibility: The esterification reaction is reversible, meaning that esters can also be converted back into their parent alcohols and carboxylic acids through hydrolysis.
Types of Esters
Esters can be classified into several categories based on their structure and origin:
1. Simple Esters: These esters are formed from simple alcohols and carboxylic acids. For example, ethyl acetate is a simple ester derived from ethanol and acetic acid.
2. Complex Esters: These esters are derived from complex alcohols or carboxylic acids, often containing multiple functional groups. An example is glycerol triacetate, which is formed from glycerol and acetic acid.
3. Fatty Acid Esters: These esters are derived from fatty acids and are commonly found in lipids. They play a crucial role in biological systems, serving as energy storage molecules and structural components of cell membranes.
4. Polymeric Esters: These esters are formed through the polymerization of monomeric esters. Polyesters, such as polyethylene terephthalate (PET), are widely used in the production of fibers, plastics, and films.
Applications of Esters
Esters have a wide range of applications across various industries:
1. Food Industry: Esters are commonly used as flavoring agents and food additives due to their pleasant aromas and tastes. For example, isoamyl acetate is responsible for the banana flavor in candies and beverages.
2. Fragrance Industry: Many esters are used in perfumes and cosmetics for their appealing scents. They are often synthesized to mimic natural fragrances found in fruits and flowers.
3. Pharmaceuticals: Esters are important in the pharmaceutical industry, where they are used as intermediates in the synthesis of various drugs. Some medications are also formulated as esters to improve their solubility and bioavailability.
4. Polymer Production: Esters are key components in the production of polymers, such as polyesters, which are used in textiles, packaging, and plastic products. PET, for example, is widely used in beverage bottles and synthetic fibers.
5. Solvents: Many esters serve as solvents in industrial applications due to their ability to dissolve a wide range of organic compounds. Ethyl acetate and butyl acetate are commonly used solvents in paints, coatings, and adhesives.
6. Biodiesel Production: Esters derived from vegetable oils or animal fats are used to produce biodiesel through transesterification. This renewable fuel source is gaining popularity as an alternative to fossil fuels.
Significance of Esters
Esters are significant for several reasons:
1. Biological Importance: Esters play crucial roles in biological systems. For example, triglycerides, which are esters of glycerol and fatty acids, serve as energy storage molecules in living organisms. Phospholipids, which are also esters, are essential components of cell membranes.
2. Environmental Impact: The use of esters as solvents and in biodiesel production can have positive environmental implications. Esters are often less toxic and more biodegradable than traditional solvents, reducing their impact on ecosystems.
3. Cultural Relevance: Esters are integral to the food and fragrance industries, influencing culinary practices and consumer products. Their pleasant aromas and flavors enhance the sensory experience of food and personal care products.
4. Economic Value: The production and use of esters contribute significantly to the economy. They are valuable commodities in various industries, and their versatility makes them essential in both industrial and consumer applications.
Conclusion
Esters are a diverse and important class of organic compounds with a wide range of applications and significance in various fields. Their unique chemical structure, properties, and formation processes make them essential in food, fragrance, pharmaceuticals, and polymer production. Understanding esters’ roles in biological systems, environmental impact, and cultural relevance highlights their importance in our daily lives.
As research continues to explore the complexities of organic chemistry and its applications, esters will remain a key focus of study, providing insights into their potential uses and benefits across different industries. Their versatility and significance underscore the importance of these compounds in both scientific and practical contexts, making them a vital area of exploration in the world of chemistry.