Electrolytic conductance is a fundamental concept in electrochemistry that describes the ability of an electrolyte solution to conduct electric current. This property is crucial in various applications, including batteries, electrolysis, and electroplating. Understanding the factors that influence electrolytic conductance is essential for optimizing these processes and improving the efficiency of electrochemical systems. This article will provide a detailed exploration of the factors affecting electrolytic conductance, including concentration, temperature, nature of the electrolyte, ion mobility, and the presence of impurities, along with illustrative explanations to enhance comprehension.
1. What is Electrolytic Conductance?
Electrolytic conductance refers to the ability of an electrolyte solution to conduct electricity. It is measured in siemens (S) and is influenced by the presence of ions in the solution. When an electric field is applied, ions move towards the electrodes, allowing current to flow. The conductance of an electrolyte solution can be expressed mathematically as:
![]()
Where:
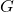 = conductance (in siemens)
= conductance (in siemens)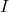 = current (in amperes)
= current (in amperes)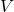 = voltage (in volts)
= voltage (in volts)
Illustrative Explanation: Imagine a water slide at a water park. The slide represents the pathway for electricity, while the water (ions) flowing down the slide represents the electric current. The more water there is (higher ion concentration), the faster the current can flow, just as more ions lead to higher conductance.
2. Factors Affecting Electrolytic Conductance
Several key factors influence the electrolytic conductance of a solution:
A. Concentration of the Electrolyte
The concentration of ions in a solution is one of the most significant factors affecting conductance. Generally, as the concentration of the electrolyte increases, the number of ions available to carry charge also increases, leading to higher conductance.
- Illustrative Explanation: Think of a crowded highway. When there are more cars (ions) on the road, traffic (current) flows more smoothly and quickly. Conversely, if there are fewer cars, traffic slows down, just as lower ion concentration results in reduced conductance.
B. Temperature
Temperature has a profound effect on electrolytic conductance. As temperature increases, the kinetic energy of the ions also increases, leading to greater mobility and, consequently, higher conductance. However, the relationship is not always linear, as the dissociation of electrolytes can also be temperature-dependent.
- Illustrative Explanation: Imagine a group of people dancing at a party. When the music (temperature) gets faster, the dancers (ions) move more energetically and can cover more ground, leading to a livelier atmosphere (higher conductance). Conversely, if the music slows down, the dancers move less, and the energy in the room decreases (lower conductance).
C. Nature of the Electrolyte
The type of electrolyte used significantly affects conductance. Strong electrolytes, which completely dissociate into ions in solution (e.g., sodium chloride, NaCl), generally exhibit higher conductance than weak electrolytes, which only partially dissociate (e.g., acetic acid, CH₃COOH).
- Illustrative Explanation: Think of strong electrolytes as a well-organized team of players in a game. Each player (ion) knows their role and contributes effectively to the team’s performance (conductance). In contrast, weak electrolytes are like a disorganized team where not all players are actively participating, leading to less effective performance.
D. Ion Mobility
Ion mobility refers to the speed at which ions move through a solution under the influence of an electric field. Factors such as ion size, charge, and the viscosity of the solvent can affect ion mobility. Smaller ions with higher charges typically move faster than larger ions with lower charges.
- Illustrative Explanation: Imagine a race between two groups of runners. The smaller, faster runners (small, highly charged ions) can navigate the course quickly, while larger, slower runners (large, less charged ions) struggle to keep up. The overall speed of the race (conductance) depends on the performance of all the runners.
E. Presence of Impurities
The presence of impurities in an electrolyte solution can significantly affect conductance. Some impurities may introduce additional ions into the solution, increasing conductance, while others may interfere with ion movement, reducing conductance.
- Illustrative Explanation: Think of a river flowing through a landscape. If the river is clear (pure electrolyte), the water flows smoothly. However, if there are obstacles (impurities) in the river, such as rocks or debris, the flow is disrupted, leading to slower movement (lower conductance). Conversely, if the impurities are additional water sources (extra ions), they can enhance the flow.
3. Mathematical Representation of Conductance
The relationship between conductance and the factors mentioned can be expressed mathematically using the following equation:
![]()
Where:
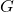 = conductance (in siemens)
= conductance (in siemens)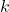 = specific conductance (a constant that depends on the nature of the electrolyte and temperature)
= specific conductance (a constant that depends on the nature of the electrolyte and temperature)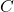 = concentration of the electrolyte (in moles per liter)
= concentration of the electrolyte (in moles per liter)
This equation highlights the direct relationship between concentration and conductance, while the specific conductance ![]() accounts for the nature of the electrolyte and temperature.
accounts for the nature of the electrolyte and temperature.
Illustrative Explanation: Imagine a recipe for a delicious dish. The specific conductance ![]() is like the recipe’s instructions, guiding you on how to combine ingredients (ions) to achieve the desired flavor (conductance). The more ingredients you add (higher concentration), the more flavorful the dish becomes.
is like the recipe’s instructions, guiding you on how to combine ingredients (ions) to achieve the desired flavor (conductance). The more ingredients you add (higher concentration), the more flavorful the dish becomes.
4. Applications of Electrolytic Conductance
Understanding the factors affecting electrolytic conductance is crucial for various applications, including:
A. Electrochemical Cells
In batteries and fuel cells, electrolytic conductance is vital for efficient energy conversion. Optimizing the concentration and temperature of the electrolyte can enhance the performance of these devices.
- Illustrative Explanation: Think of a battery as a race car. The electrolyte is the fuel that powers the car. By ensuring the right fuel mixture (concentration and temperature), the car can perform at its best, just as optimizing electrolytic conductance improves battery efficiency.
B. Water Quality Testing
Conductance measurements are often used to assess the quality of water. Higher conductance can indicate the presence of dissolved salts and impurities, which can affect water quality.
- Illustrative Explanation: Imagine testing the clarity of a swimming pool. If the water is clear (low conductance), it is likely clean and safe for swimming. However, if the water is murky (high conductance), it may contain unwanted substances, indicating a need for treatment.
C. Electroplating
In electroplating processes, controlling the conductance of the electrolyte solution is essential for achieving uniform deposition of metal coatings on surfaces.
- Illustrative Explanation: Think of electroplating as painting a wall. The electrolyte solution is like the paint, and the conductance determines how evenly the paint is applied. If the paint is too thick (high conductance), it may run; if it’s too thin (low conductance), it may not cover well.
5. Conclusion
Electrolytic conductance is a critical property that influences various electrochemical processes and applications. Understanding the factors that affect conductance—such as concentration, temperature, nature of the electrolyte, ion mobility, and the presence of impurities—enables scientists and engineers to optimize systems for improved performance. By grasping these concepts, we can better appreciate the intricacies of electrochemistry and its applications in our daily lives, from the batteries that power our devices to the water we drink. As we continue to explore the world of electrolytic conductance, we gain valuable insights into the behavior of ions and the fundamental principles that govern electrical conductivity in solutions.