The Finkelstein reaction is a well-known organic reaction that involves the exchange of halogens in alkyl halides, typically resulting in the conversion of an alkyl chloride or bromide into an alkyl iodide. Named after the chemist Hugo Finkelstein, who first described the reaction in 1935, this reaction is significant in organic synthesis due to its utility in preparing iodides, which are often more reactive and useful in subsequent chemical transformations. This article delves into the mechanisms, conditions, applications, and significance of the Finkelstein reaction, providing a thorough understanding of this important chemical process.
Mechanism of the Finkelstein Reaction
Understanding the mechanism of the Finkelstein reaction is crucial for grasping how halogen exchange occurs. The reaction typically follows an SN2 (nucleophilic substitution bimolecular) mechanism, characterized by a one-step process where the nucleophile attacks the electrophile, leading to the simultaneous displacement of the leaving group.
1. Nucleophilic Attack
In the Finkelstein reaction, the nucleophile is usually iodide ion (I⁻), which is a strong nucleophile. The reaction begins when the iodide ion attacks the carbon atom of the alkyl halide (R-X), where X is the leaving group (chlorine or bromine). This attack occurs at the carbon atom that is bonded to the halogen, resulting in the formation of a transition state.
Illustrative Explanation: Imagine the nucleophilic attack as a game of tag. The iodide ion is like a player trying to tag the carbon atom (the target), while the leaving group (X) is being pushed away. As the iodide approaches, the carbon atom is temporarily in a state of flux, balancing between the incoming iodide and the outgoing halogen.
2. Formation of the Transition State
During the nucleophilic attack, a transition state is formed where the carbon atom is simultaneously bonded to both the iodide ion and the leaving group. This transition state is unstable and represents a high-energy configuration.
Illustrative Explanation: Visualize the transition state as a tightrope walker balancing precariously. Just as the walker must maintain balance to avoid falling, the carbon atom in the transition state is caught between the incoming iodide and the departing halogen, creating a moment of instability.
3. Departure of the Leaving Group
As the nucleophilic attack progresses, the bond between the carbon atom and the leaving group weakens, ultimately leading to the departure of the leaving group (X). Once the leaving group is expelled, the carbon atom is now bonded to the iodide ion, resulting in the formation of the alkyl iodide (R-I).
Illustrative Explanation: Think of the departure of the leaving group as a relay race. The leaving group (X) is like a runner passing the baton to the incoming iodide (I⁻). Once the baton is passed, the leaving group exits the race, and the iodide takes its place, completing the reaction.
Overall Reaction
The overall reaction can be summarized as follows:
![]()
Where:
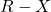 is the alkyl halide (chloride or bromide).
is the alkyl halide (chloride or bromide).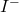 is the iodide ion (nucleophile).
is the iodide ion (nucleophile).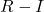 is the resulting alkyl iodide.
is the resulting alkyl iodide.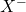 is the expelled leaving group (chloride or bromide).
is the expelled leaving group (chloride or bromide).
Conditions for the Finkelstein Reaction
The Finkelstein reaction requires specific conditions to proceed efficiently. Understanding these conditions is essential for successful implementation in the laboratory.
1. Solvent Choice
The choice of solvent plays a crucial role in the Finkelstein reaction. Polar aprotic solvents, such as acetone or DMSO (dimethyl sulfoxide), are commonly used because they solvate the nucleophile (iodide ion) while not stabilizing the leaving group too much. This enhances the nucleophilicity of the iodide ion, facilitating the reaction.
Illustrative Explanation: Imagine the solvent as a supportive coach. Just as a coach encourages players to perform at their best, a polar aprotic solvent helps the iodide ion remain active and ready to attack the alkyl halide.
2. Temperature
The reaction is typically carried out at room temperature or slightly elevated temperatures. Higher temperatures can increase the reaction rate but may also lead to side reactions or decomposition of sensitive substrates.
Illustrative Explanation: Think of temperature as the energy level of a dance party. Just as a lively atmosphere can energize dancers, a moderate temperature can enhance the reaction rate without causing chaos.
3. Reactant Concentration
The concentration of the reactants can also influence the reaction rate. Higher concentrations of the alkyl halide and iodide ion can lead to increased reaction rates, as there are more reactant molecules available for interaction.
Illustrative Explanation: Visualize reactant concentration as the number of players on a sports team. Just as a larger team can increase the chances of scoring, higher concentrations of reactants can enhance the likelihood of successful collisions leading to the reaction.
Applications of the Finkelstein Reaction
The Finkelstein reaction has several important applications in organic synthesis and industrial chemistry. Understanding these applications highlights the significance of this reaction in various fields.
1. Synthesis of Iodinated Compounds
One of the primary applications of the Finkelstein reaction is the synthesis of iodinated compounds, which are often more reactive than their chlorinated or brominated counterparts. Iodinated compounds are valuable intermediates in the synthesis of pharmaceuticals, agrochemicals, and other organic compounds.
Illustrative Explanation: Think of iodinated compounds as the secret ingredients in a recipe. Just as certain ingredients can elevate a dish, iodinated compounds can enhance the reactivity and utility of organic molecules in chemical synthesis.
2. Preparation of Radiolabeled Compounds
The Finkelstein reaction is also used in the preparation of radiolabeled compounds for use in medical imaging and research. Iodine-125 and iodine-131 are commonly used isotopes in radiolabeling, and the Finkelstein reaction can facilitate their incorporation into organic molecules.
Illustrative Explanation: Visualize radiolabeled compounds as beacons in a dark room. Just as a beacon can guide someone to safety, radiolabeled compounds help researchers track biological processes and diagnose medical conditions.
3. Organic Synthesis
The Finkelstein reaction is a valuable tool in organic synthesis, allowing chemists to modify existing compounds and create new ones. It is often used in multi-step synthesis processes, where the introduction of iodine can lead to further transformations.
Illustrative Explanation: Consider organic synthesis as a complex puzzle. Just as each piece must fit together to complete the picture, the Finkelstein reaction allows chemists to strategically introduce iodine into molecules, facilitating the assembly of more complex structures.
Advantages and Limitations of the Finkelstein Reaction
While the Finkelstein reaction offers several advantages, it also has limitations that must be considered in practical applications.
Advantages
1. Simplicity: The Finkelstein reaction is relatively straightforward and can be performed with common reagents and equipment, making it accessible for many laboratories.
2. High Yield: The reaction typically proceeds with high yields, especially when optimal conditions are maintained, making it an efficient method for synthesizing iodinated compounds.
3. Versatility: The Finkelstein reaction can be applied to a wide range of alkyl halides, allowing for the synthesis of various iodinated products.
Illustrative Explanation: Think of the advantages of the Finkelstein reaction as a well-oiled machine. Just as a machine operates smoothly and efficiently, the reaction’s simplicity, high yield, and versatility make it a valuable tool in organic chemistry.
Limitations
1. Substrate Limitations: The reaction is most effective with primary and some secondary alkyl halides. Tertiary alkyl halides may undergo elimination reactions instead of substitution, limiting the scope of the reaction.
2. Competing Reactions: In some cases, competing reactions, such as elimination or rearrangement, can occur, leading to undesired products.
3. Iodide Availability: The availability and cost of iodide sources can be a limitation in some regions, potentially affecting the practicality of the reaction.
Illustrative Explanation: Consider the limitations of the Finkelstein reaction as obstacles on a racetrack. Just as racers must navigate around obstacles to reach the finish line, chemists must be aware of substrate limitations and competing reactions to achieve successful outcomes.
Conclusion
In conclusion, the Finkelstein reaction is a fundamental process in organic chemistry that facilitates the exchange of halogens in alkyl halides, resulting in the formation of iodinated compounds. Through its well-defined mechanism, specific conditions, and diverse applications, the Finkelstein reaction plays a crucial role in organic synthesis, pharmaceutical development, and radiolabeling. While it offers several advantages, including simplicity and high yield, it also presents limitations that chemists must navigate. By understanding the intricacies of the Finkelstein reaction, researchers and practitioners can harness its potential to create valuable compounds and advance the field of organic chemistry. As we continue to explore the complexities of chemical reactions, the Finkelstein reaction remains a testament to the elegance and utility of organic synthesis in scientific discovery and innovation.