Heat capacity is a fundamental concept in thermodynamics and physical chemistry that describes the ability of a substance to absorb heat energy. It plays a crucial role in understanding how materials respond to changes in temperature, which is essential in various scientific and engineering applications. This article will delve into the definition of heat capacity, its types, the factors affecting it, its significance in real-world applications, and illustrative explanations to clarify each concept.
What is Heat Capacity?
Definition
Heat capacity is defined as the amount of heat energy required to raise the temperature of a given quantity of a substance by one degree Celsius (°C) or one Kelvin (K). It is an extensive property, meaning it depends on the amount of substance present.
- Mathematical Expression: The heat capacity (C) can be expressed mathematically as:
![]()
where:
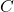 = heat capacity (in joules per degree Celsius, J/°C)
= heat capacity (in joules per degree Celsius, J/°C)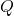 = heat energy added or removed (in joules, J)
= heat energy added or removed (in joules, J)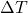 = change in temperature (in degrees Celsius or Kelvin, °C or K)
= change in temperature (in degrees Celsius or Kelvin, °C or K)
Illustrative Explanation
Imagine a sponge soaking up water. Just as the amount of water a sponge can absorb depends on its size, the heat capacity of a substance depends on its mass and the amount of heat it can store. If you pour a small amount of water onto a small sponge, it will soak it up quickly, but a larger sponge will take longer to reach the same saturation point. Similarly, different substances have different heat capacities, affecting how they respond to heat.
Types of Heat Capacity
Heat capacity can be categorized into two main types based on the conditions under which it is measured:
1. Specific Heat Capacity (c)
Specific heat capacity is the amount of heat required to raise the temperature of one unit mass of a substance by one degree Celsius (°C) or one Kelvin (K). It is an intensive property, meaning it does not depend on the amount of substance present.
- Mathematical Expression: The specific heat capacity can be expressed as:
![]()
where:
 = specific heat capacity (in joules per kilogram per degree Celsius, J/(kg·°C))
= specific heat capacity (in joules per kilogram per degree Celsius, J/(kg·°C))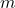 = mass of the substance (in kilograms, kg)
= mass of the substance (in kilograms, kg)- Illustrative Explanation: Think of a pot of water on the stove. If you want to heat the water, the specific heat capacity tells you how much energy you need to add to raise the temperature of a specific amount of water. For example, water has a high specific heat capacity (about 4.18 J/(g·°C)), meaning it requires a significant amount of energy to increase its temperature, which is why it takes time to boil.
2. Molar Heat Capacity (C_m)
Molar heat capacity is the amount of heat required to raise the temperature of one mole of a substance by one degree Celsius (°C) or one Kelvin (K). Like specific heat capacity, it is also an intensive property.
- Mathematical Expression: The molar heat capacity can be expressed as:
![]()
where:
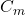 = molar heat capacity (in joules per mole per degree Celsius, J/(mol·°C))
= molar heat capacity (in joules per mole per degree Celsius, J/(mol·°C))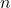 = number of moles of the substance (in moles, mol)
= number of moles of the substance (in moles, mol)- Illustrative Explanation: Imagine a bag of flour. If you want to bake a cake, the molar heat capacity tells you how much energy is needed to raise the temperature of a specific number of moles of flour. Just as different types of flour may require different amounts of energy to heat, different substances have different molar heat capacities.
Factors Affecting Heat Capacity
Several factors influence the heat capacity of a substance:
1. Nature of the Substance
Different materials have different heat capacities due to their molecular structure and bonding. For example, metals generally have lower specific heat capacities than water.
- Illustrative Explanation: Think of a metal frying pan and a pot of water. The frying pan heats up quickly because it has a low specific heat capacity, while the water takes longer to heat up due to its high specific heat capacity. This is why you can quickly sear food in a hot pan, but it takes time to bring water to a boil.
2. Phase of the Substance
The heat capacity of a substance varies depending on its phase (solid, liquid, or gas). Generally, gases have higher heat capacities than liquids, and liquids have higher heat capacities than solids.
- Illustrative Explanation: Imagine heating ice, water, and steam. When you heat ice (solid), it requires energy to melt into water (liquid) before its temperature can rise. Similarly, heating water to turn it into steam (gas) requires additional energy. Each phase change involves different heat capacities, illustrating how the state of matter affects heat absorption.
3. Temperature
The heat capacity of some substances can change with temperature. For example, the specific heat capacity of water decreases as it approaches its boiling point.
- Illustrative Explanation: Consider a balloon filled with air. As you heat the air inside the balloon, it expands. Similarly, as the temperature of a substance increases, its ability to absorb heat may change, affecting its heat capacity.
Significance of Heat Capacity
Understanding heat capacity is essential in various fields, including:
1. Thermal Management
In engineering and design, knowledge of heat capacity is crucial for thermal management in systems such as engines, electronics, and buildings. Proper heat capacity calculations help ensure that materials can withstand temperature changes without failure.
- Illustrative Explanation: Think of a car engine. Just as a mechanic needs to know how much heat the engine can handle to prevent overheating, engineers must understand the heat capacity of materials used in construction to ensure they can manage temperature fluctuations effectively.
2. Climate Science
Heat capacity plays a significant role in climate science, particularly in understanding how oceans and land masses absorb and release heat. The high heat capacity of water helps regulate global temperatures and climate patterns.
- Illustrative Explanation: Imagine a large sponge soaking up water. Just as the sponge can absorb a lot of water without overflowing, the oceans can absorb and store vast amounts of heat, moderating the Earth’s climate and influencing weather patterns.
3. Cooking and Food Science
In cooking, understanding the heat capacity of different ingredients helps chefs control cooking times and temperatures, ensuring that food is cooked evenly and thoroughly.
- Illustrative Explanation: Consider baking a cake. Just as a chef needs to know how long to bake the cake based on the heat capacity of the batter, understanding heat capacity helps ensure that the cake rises and cooks properly without burning.
Conclusion
Heat capacity is a fundamental concept that describes how substances absorb and store heat energy. By understanding the different types of heat capacity, the factors that influence it, and its significance in various applications, we can better appreciate the role of heat capacity in our daily lives and the natural world. Whether in engineering, climate science, or cooking, heat capacity is a critical factor that influences how materials respond to temperature changes. As we continue to explore the intricacies of heat capacity, we unlock new possibilities for innovation and understanding in science and technology.