Henry’s Law is a fundamental principle in physical chemistry that describes the behavior of gases dissolved in liquids. Named after the British chemist William Henry, who formulated the law in 1803, it provides a quantitative relationship between the concentration of a gas in a liquid and the partial pressure of that gas above the liquid. This law is crucial for understanding various phenomena in fields such as environmental science, biology, and engineering. This article will provide a detailed examination of Henry’s Law, including its definition, mathematical expression, applications, limitations, and illustrative explanations to enhance comprehension.
1. Overview of Henry’s Law
Definition: Henry’s Law states that at a constant temperature, the amount of gas that dissolves in a liquid is directly proportional to the partial pressure of that gas in equilibrium with the liquid. In simpler terms, if the pressure of a gas above a liquid increases, more of that gas will dissolve in the liquid.
Illustrative Explanation: Imagine a soda can filled with carbonated water. When you open the can, the pressure inside decreases, and you hear a “hiss” as the gas escapes. This is a practical demonstration of Henry’s Law: when the pressure of carbon dioxide (CO₂) is reduced, the amount of CO₂ that can remain dissolved in the liquid also decreases, leading to the formation of bubbles.
2. Mathematical Expression
The mathematical expression of Henry’s Law can be represented as:
![]()
Where:
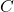 = concentration of the dissolved gas (usually in moles per liter, mol/L)
= concentration of the dissolved gas (usually in moles per liter, mol/L)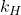 = Henry’s Law constant (specific to each gas-liquid pair and temperature)
= Henry’s Law constant (specific to each gas-liquid pair and temperature)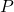 = partial pressure of the gas above the liquid (in atmospheres, atm)
= partial pressure of the gas above the liquid (in atmospheres, atm)
Illustrative Explanation: Think of this equation as a balance scale. On one side, you have the concentration of the dissolved gas, and on the other side, you have the pressure of the gas. If you increase the pressure (adding weight to one side), the concentration of the dissolved gas must also increase to maintain balance.
3. Applications of Henry’s Law
Henry’s Law has several important applications across various fields:
A. Carbonated Beverages
- Definition: In the production of carbonated drinks, carbon dioxide is dissolved in the liquid under high pressure. When the bottle or can is opened, the pressure drops, and the gas escapes, creating bubbles.
- Illustrative Explanation: Imagine a fizzy drink as a party in a bottle. The high pressure keeps the guests (carbon dioxide molecules) packed tightly together in the liquid. When the pressure is released, the guests rush out to join the party, forming bubbles and fizz.
B. Aquatic Life
- Definition: Henry’s Law is crucial for understanding how gases like oxygen and carbon dioxide dissolve in water, which is essential for the survival of aquatic organisms.
- Illustrative Explanation: Picture a fish swimming in a lake. The fish relies on dissolved oxygen in the water to breathe. According to Henry’s Law, if the atmospheric pressure increases (for example, during a storm), more oxygen will dissolve in the water, benefiting the fish. Conversely, if the pressure decreases, the oxygen levels may drop, potentially harming aquatic life.
C. Environmental Science
- Definition: Henry’s Law is used to model the behavior of pollutants in water bodies, helping scientists understand how gases like volatile organic compounds (VOCs) behave in aquatic environments.
- Illustrative Explanation: Think of a lake as a sponge soaking up gases from the air. If the concentration of a pollutant in the air increases (higher pressure), more of that pollutant will dissolve in the water, similar to how a sponge absorbs more water when pressed. This understanding helps in assessing the impact of pollution on ecosystems.
D. Medical Applications
- Definition: In medicine, Henry’s Law is applied in hyperbaric oxygen therapy, where patients breathe pure oxygen at higher pressures to increase the amount of oxygen dissolved in their blood.
- Illustrative Explanation: Imagine a diver coming up from deep underwater. As they ascend, the pressure decreases, and if they don’t exhale properly, nitrogen bubbles can form in their bloodstream, causing decompression sickness. Hyperbaric therapy uses Henry’s Law to ensure that patients can safely absorb more oxygen under controlled high-pressure conditions.
4. Limitations of Henry’s Law
While Henry’s Law is a valuable principle, it has limitations:
A. Ideal Conditions
- Definition: Henry’s Law assumes ideal behavior, meaning it applies best to dilute solutions and low pressures. At high concentrations or pressures, deviations from the law can occur.
- Illustrative Explanation: Think of Henry’s Law as a set of guidelines for a recipe. Just as a recipe works best with specific ingredients and conditions, Henry’s Law is most accurate under ideal conditions. If you add too many ingredients (high concentrations), the results may not match the expected outcome.
B. Temperature Dependence
- Definition: The Henry’s Law constant (
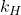 ) varies with temperature. As temperature increases, the solubility of gases typically decreases, leading to lower concentrations at higher temperatures.
) varies with temperature. As temperature increases, the solubility of gases typically decreases, leading to lower concentrations at higher temperatures. - Illustrative Explanation: Imagine a pot of water on the stove. As you heat the water, the steam (gas) escapes more quickly. Similarly, as the temperature rises, gases become less soluble in liquids, which can affect the predictions made by Henry’s Law.
5. Conclusion
In conclusion, Henry’s Law is a fundamental principle in physical chemistry that describes the relationship between the concentration of a gas in a liquid and the partial pressure of that gas above the liquid. By understanding its mathematical expression, applications, and limitations, we can appreciate its significance in various scientific fields and everyday life. Through illustrative explanations, we can visualize how changes in pressure affect gas solubility, reinforcing the concept that gases and liquids interact in complex ways. Whether in carbonated beverages, aquatic ecosystems, environmental science, or medical applications, Henry’s Law remains a crucial tool for understanding the behavior of gases in liquids, contributing to our knowledge of chemistry and its real-world implications.