The Law of Conservation of Energy is a fundamental principle in physics that states that energy cannot be created or destroyed; it can only be transformed from one form to another. This law is a cornerstone of both classical and modern physics and has profound implications across various scientific disciplines, including mechanics, thermodynamics, and electromagnetism. Understanding this law is essential for analyzing physical systems and processes in the natural world. This article will provide a detailed exploration of the Law of Conservation of Energy, including its definition, historical context, mathematical formulation, applications, and illustrative explanations of each concept.
Definition of the Law of Conservation of Energy
The Law of Conservation of Energy asserts that the total energy of an isolated system remains constant over time. While energy can change forms—such as from kinetic energy to potential energy or from thermal energy to mechanical energy—the total amount of energy within the system remains unchanged.
- Illustrative Explanation: Consider a pendulum swinging back and forth. At the highest point of its swing, the pendulum has maximum potential energy and minimal kinetic energy. As it swings down, potential energy is converted into kinetic energy, reaching maximum kinetic energy at the lowest point of the swing. As it rises again, kinetic energy is converted back into potential energy. Throughout this motion, the total energy of the pendulum remains constant, illustrating the conservation of energy.
Historical Context
The concept of energy conservation has evolved over centuries. Early ideas about energy can be traced back to the work of scientists such as Galileo and Newton, who studied motion and forces. However, the formalization of the Law of Conservation of Energy emerged in the 19th century with the development of thermodynamics and the work of key figures such as:
1. Julius Robert von Mayer: In 1842, Mayer proposed that energy is conserved in mechanical processes and introduced the idea that heat is a form of energy.
2. James Prescott Joule: Joule conducted experiments demonstrating the equivalence of mechanical work and heat, leading to the formulation of the first law of thermodynamics, which incorporates the conservation of energy.
3. Hermann von Helmholtz: In 1847, Helmholtz published a paper that explicitly stated the principle of conservation of energy, solidifying its status as a fundamental law of physics.
Mathematical Formulation
The Law of Conservation of Energy can be mathematically expressed in various forms, depending on the context. In a closed system, the total energy ![]() can be represented as:
can be represented as:
![]()
Where:
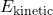 is the kinetic energy of the system.
is the kinetic energy of the system.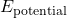 is the potential energy stored in the system.
is the potential energy stored in the system.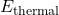 is the thermal energy (heat) within the system.
is the thermal energy (heat) within the system.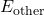 represents any other forms of energy present, such as chemical or electrical energy.
represents any other forms of energy present, such as chemical or electrical energy.
The principle states that:
![]()
This means that any change in the total energy of the system is zero, indicating that energy is conserved.
Applications of the Law of Conservation of Energy
The Law of Conservation of Energy has numerous applications across various fields of science and engineering:
1. Mechanical Systems: In mechanics, the conservation of energy is used to analyze the motion of objects. For example, in roller coasters, the potential energy at the top of a hill is converted into kinetic energy as the coaster descends, allowing for thrilling rides.
2. Thermodynamics: In thermodynamic processes, the conservation of energy is fundamental to understanding heat engines, refrigerators, and other systems. The first law of thermodynamics states that the change in internal energy of a system is equal to the heat added to the system minus the work done by the system.
3. Electrical Systems: In electrical circuits, the conservation of energy applies to the flow of electric current. The energy supplied by a power source is equal to the energy consumed by the components in the circuit, such as resistors and capacitors.
4. Chemical Reactions: In chemistry, the conservation of energy is observed in exothermic and endothermic reactions. In exothermic reactions, energy is released as chemical bonds are formed, while in endothermic reactions, energy is absorbed to break chemical bonds.
5. Environmental Science: The conservation of energy principle is crucial in understanding ecological systems and energy flow within ecosystems. Energy from the sun is captured by plants through photosynthesis and transferred through food chains, illustrating energy transformation and conservation in nature.
Illustrative Examples of Energy Conservation
1. Pendulum Motion: As previously mentioned, a pendulum demonstrates energy conservation through the conversion of potential and kinetic energy. At the highest point, the pendulum has maximum potential energy. As it swings down, this potential energy is converted into kinetic energy, which is maximum at the lowest point. As it swings back up, kinetic energy is converted back into potential energy, illustrating the continuous transformation of energy while the total energy remains constant.
2. Roller Coaster: In a roller coaster, the car gains potential energy as it climbs to the top of a hill. At the peak, it has maximum potential energy and minimal kinetic energy. As it descends, potential energy is converted into kinetic energy, causing the car to speed up. At the bottom of the hill, kinetic energy is at its maximum, and as the car climbs the next hill, kinetic energy is converted back into potential energy. Throughout the ride, the total mechanical energy of the system remains constant, demonstrating the conservation of energy.
3. Heating Water: When heating water in a kettle, electrical energy is converted into thermal energy. The electrical energy supplied to the kettle causes the water molecules to vibrate more rapidly, increasing the water’s temperature. The total energy before and after heating remains constant, as the electrical energy is transformed into thermal energy.
4. Bouncing Ball: When a ball is dropped, it converts potential energy into kinetic energy as it falls. Upon hitting the ground, some kinetic energy is transformed into sound and thermal energy due to the impact, while the rest is converted back into potential energy as the ball bounces back up. The total energy of the system is conserved, although some energy is dissipated as sound and heat.
Conclusion
In conclusion, the Law of Conservation of Energy is a fundamental principle that underpins much of physics and our understanding of the natural world. It states that energy cannot be created or destroyed, only transformed from one form to another. This law has profound implications across various scientific disciplines, from mechanics and thermodynamics to chemistry and environmental science. By understanding the conservation of energy, we can analyze and predict the behavior of physical systems, optimize energy use, and appreciate the interconnectedness of energy transformations in nature. As we continue to explore the universe, the Law of Conservation of Energy remains a guiding principle that helps us make sense of the complex interactions that govern our world.