The line spectrum of hydrogen is a fundamental concept in atomic physics and quantum mechanics that provides critical insights into the structure of atoms and the nature of light. When hydrogen gas is energized, it emits light at specific wavelengths, resulting in a spectrum composed of distinct lines. This phenomenon is not only pivotal for understanding atomic structure but also serves as a cornerstone for various applications in spectroscopy, astrophysics, and quantum theory. This article aims to provide an exhaustive overview of the line spectrum of hydrogen, including its definition, formation, characteristics, implications, and illustrative explanations of each concept to enhance understanding.
Definition of Line Spectrum
1. Basic Definition:
- A line spectrum, also known as an emission spectrum, is a spectrum that consists of discrete lines, each corresponding to a specific wavelength of light emitted by an atom or molecule. In the case of hydrogen, the line spectrum arises from electronic transitions between energy levels within the hydrogen atom.
Illustrative Explanation: Imagine a musical performance where each musician (electron) plays a specific note (wavelength) at a precise moment. The resulting sound (spectrum) is not a continuous melody but a series of distinct notes (lines) that create a unique composition (line spectrum).
2. Hydrogen Atom:
- The hydrogen atom is the simplest atom, consisting of one proton and one electron. Its simplicity makes it an ideal candidate for studying atomic structure and the behavior of electrons in energy levels.
Illustrative Example: Think of the hydrogen atom as a single light bulb (electron) orbiting around a central power source (proton). The light bulb can only shine at certain brightness levels (energy levels), creating specific colors (wavelengths) when it emits light.
Formation of the Line Spectrum
1. Energy Levels:
- Electrons in an atom occupy specific energy levels or orbitals. In hydrogen, these energy levels are quantized, meaning that the electron can only exist in certain allowed states. The energy levels are denoted by the principal quantum number
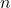 , where
, where 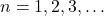 .
.
Illustrative Explanation: Imagine a staircase (energy levels) where each step represents a specific height (energy level). The electron can only stand on the steps (allowed energy levels) and cannot exist in between them (quantization).
2. Excitation and Emission:
- When hydrogen gas is energized (for example, by heating or applying an electric current), the electron absorbs energy and moves to a higher energy level (excitation). When the electron returns to a lower energy level, it releases energy in the form of light. The wavelength of this emitted light corresponds to the difference in energy between the two levels.
Illustrative Example: Picture a child (electron) jumping from one step (energy level) to a higher step (excited state) after receiving a push (energy absorption). When the child jumps back down to a lower step (lower energy level), they release energy in the form of a sound (light emission), which can be heard at specific pitches (wavelengths).
3. Balmer Series:
- The visible line spectrum of hydrogen is primarily represented by the Balmer series, which includes transitions from higher energy levels to the second energy level (
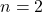 ). The wavelengths of light emitted in this series correspond to visible colors, such as red, green, and blue.
). The wavelengths of light emitted in this series correspond to visible colors, such as red, green, and blue.
Illustrative Explanation: Imagine a rainbow (spectrum) created by a prism. Each color in the rainbow represents a different note played by the child (electron) as they jump down from various heights (energy levels) to the second step (Balmer series).
Characteristics of the Line Spectrum
1. Discrete Lines:
- The line spectrum of hydrogen consists of distinct lines, each representing a specific wavelength of light. These lines are sharp and well-defined, indicating that the emitted light corresponds to specific energy transitions.
Illustrative Explanation: Think of a painter (hydrogen atom) creating a series of precise strokes (lines) on a canvas (spectrum). Each stroke represents a specific color (wavelength) that stands out clearly against the background.
2. Wavelengths and Frequencies:
- Each line in the spectrum corresponds to a specific wavelength and frequency of light. The relationship between wavelength (
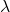 ) and frequency (
) and frequency (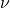 ) is given by the equation
) is given by the equation 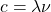 , where
, where  is the speed of light.
is the speed of light.
Illustrative Example: Imagine a race (light) where each runner (wavelength) has a different speed (frequency). The faster runners (shorter wavelengths) cross the finish line first, while the slower ones (longer wavelengths) take longer, creating a distinct order (line spectrum).
3. Rydberg Formula:
- The wavelengths of the lines in the hydrogen spectrum can be calculated using the Rydberg formula:
![]()
where ![]() is the Rydberg constant,
is the Rydberg constant, ![]() is the lower energy level, and
is the lower energy level, and ![]() is the higher energy level. This formula allows for the prediction of the wavelengths of the spectral lines.
is the higher energy level. This formula allows for the prediction of the wavelengths of the spectral lines.
Illustrative Explanation: Think of a recipe (Rydberg formula) that tells you how to mix ingredients (energy levels) to create a specific dish (wavelength). By following the recipe, you can predict the outcome (wavelength) based on the ingredients used (energy levels).
Implications of the Line Spectrum
1. Atomic Structure:
- The line spectrum of hydrogen provides crucial evidence for the quantized nature of atomic energy levels. It supports the Bohr model of the atom, which describes electrons as occupying specific orbits around the nucleus.
Illustrative Explanation: Imagine a solar system (atom) where planets (electrons) orbit the sun (nucleus) at fixed distances (energy levels). The distinct paths (orbits) of the planets illustrate the quantized nature of their positions.
2. Spectroscopy:
- The study of the line spectrum is fundamental to spectroscopy, a technique used to analyze the composition of substances. By examining the emitted or absorbed light, scientists can identify elements and compounds based on their unique spectral lines.
Illustrative Example: Picture a detective (scientist) using a magnifying glass (spectroscopy) to examine clues (spectral lines) left at a crime scene (substance). Each clue provides valuable information about what happened (composition of the substance).
3. Astrophysics:
- The line spectrum of hydrogen is essential in astrophysics for understanding the composition and behavior of stars and galaxies. By analyzing the light emitted from celestial bodies, astronomers can determine their chemical composition, temperature, and motion.
Illustrative Explanation: Imagine an astronomer as a space explorer using a telescope (spectroscopy) to observe distant stars (celestial bodies). By analyzing the light from these stars, the astronomer can uncover secrets about their nature and behavior, much like reading a book about their history.
Applications of the Line Spectrum
1. Chemical Analysis:
- The line spectrum is widely used in chemical analysis to identify elements in a sample. Techniques such as atomic emission spectroscopy and flame tests rely on the unique spectral lines of elements to determine their presence and concentration.
Illustrative Explanation: Think of a chef (chemist) using a special spice (element) that has a unique flavor (spectral line). By tasting the dish (analyzing the sample), the chef can identify which spices are present based on their distinct flavors.
2. Laser Technology:
- The principles of the line spectrum are applied in laser technology, where specific wavelengths of light are emitted through stimulated emission. Lasers are used in various applications, including medicine, telecommunications, and manufacturing.
Illustrative Example: Imagine a concert (laser technology) where a singer (electron) performs a specific song (wavelength) that resonates with the audience (stimulated emission). The clarity and intensity of the performance (laser light) depend on the singer’s ability to hit the right notes (specific wavelengths).
3. Quantum Mechanics:
- The line spectrum of hydrogen is a key example in quantum mechanics, illustrating the quantization of energy levels and the behavior of electrons in atoms. It serves as a foundational concept for understanding more complex atomic systems.
Illustrative Explanation: Picture a puzzle (quantum mechanics) where each piece (energy level) fits together to create a complete picture (atomic behavior). The line spectrum of hydrogen provides the first pieces of the puzzle, helping to reveal the larger image of atomic structure.
Conclusion
The line spectrum of hydrogen is a fundamental concept that provides critical insights into atomic structure, the nature of light, and the behavior of electrons. By exploring its definition, formation, characteristics, implications, and applications, we gain valuable insights into the behavior of hydrogen and other elements. Just as a skilled conductor leads an orchestra to create a harmonious performance, the interplay of energy levels and electronic transitions orchestrates the emission of light, allowing us to predict and control atomic behavior. By mastering these concepts, we equip ourselves with the knowledge to analyze, predict, and influence chemical behavior, enhancing our understanding of chemistry and its applications in various fields. Whether in chemical analysis, astrophysics, or quantum mechanics, the principles of the line spectrum of hydrogen are integral to the functioning of our world and our daily experiences.