Mass is a fundamental property of matter that plays a crucial role in various fields of science, including physics, chemistry, and engineering. It is a measure of the amount of matter in an object and is often confused with weight, which is the force exerted by gravity on that mass. This extensive article will delve into the definition of mass, its types, units of measurement, the distinction between mass and weight, the role of mass in physics, and its applications in various scientific fields, providing illustrative explanations for each concept.
Definition of Mass
Mass is defined as a measure of the amount of matter in an object. It is an intrinsic property that does not change regardless of the object’s location in the universe. Mass is a scalar quantity, meaning it has magnitude but no direction. It is a fundamental characteristic of physical objects and is crucial for understanding various physical phenomena.
Types of Mass
Mass can be categorized into two primary types:
1. Inertial Mass: Inertial mass is a measure of an object’s resistance to acceleration when a force is applied. It quantifies how much an object will accelerate in response to a given force, according to Newton’s second law of motion, which states:
![]()
Where:
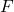 is the force applied,
is the force applied,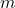 is the inertial mass,
is the inertial mass, is the acceleration produced.
is the acceleration produced.
Illustrative Explanation: Imagine pushing two objects: a small toy car and a heavy truck. When you apply the same force to both, the toy car (with a smaller inertial mass) accelerates much more than the truck (with a larger inertial mass). This illustrates how inertial mass determines an object’s response to applied forces.
2. Gravitational Mass: Gravitational mass is a measure of the strength of the gravitational force experienced by an object in a gravitational field. It determines how much gravitational force an object will experience due to the presence of other masses, such as the Earth. The gravitational force can be expressed using Newton’s law of universal gravitation:
![]()
Where:
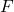 is the gravitational force,
is the gravitational force,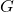 is the gravitational constant,
is the gravitational constant,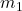 and
and 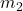 are the masses of the two objects,
are the masses of the two objects, is the distance between the centers of the two masses.
is the distance between the centers of the two masses.
Illustrative Explanation: Consider two objects, a feather and a bowling ball, both dropped from the same height in a vacuum. Despite their different masses, they fall at the same rate due to the equivalence of inertial and gravitational mass, demonstrating that gravitational mass determines the force of gravity acting on an object.
Units of Measurement
Mass is measured in various units, depending on the context and the system of measurement used:
1. Kilogram (kg): The kilogram is the base unit of mass in the International System of Units (SI). It is defined as the mass of a specific platinum-iridium cylinder kept at the International Bureau of Weights and Measures in France.
Illustrative Explanation: When you weigh an object on a scale, the reading in kilograms indicates its mass. For example, a bag of flour weighing 2 kg has a mass of 2 kilograms, which is a measure of the amount of matter it contains.
2. Gram (g): The gram is a smaller unit of mass, where 1 kilogram equals 1,000 grams. It is commonly used in chemistry and cooking.
Illustrative Explanation: A typical apple might weigh around 150 grams. This measurement indicates the mass of the apple, which is a measure of the matter it contains.
3. Metric Ton (tonne): A metric ton is equal to 1,000 kilograms and is often used to measure larger masses, such as vehicles or shipping containers.
Illustrative Explanation: A large truck might have a mass of 3 metric tons, indicating that it contains a significant amount of matter.
4. Pound (lb): In the Imperial system, mass is often measured in pounds. One kilogram is approximately equal to 2.20462 pounds.
Illustrative Explanation: If a person weighs 150 pounds, this measurement indicates their mass in the Imperial system, which can be converted to kilograms for scientific purposes.
Distinction Between Mass and Weight
It is essential to distinguish between mass and weight, as they are often confused:
- Mass: As previously defined, mass is a measure of the amount of matter in an object and is constant regardless of location. It is measured in kilograms, grams, or other units of mass.
- Weight: Weight is the force exerted on an object due to gravity. It depends on both the mass of the object and the strength of the gravitational field acting on it. Weight can be calculated using the formula:
![]()
Where:
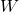 is the weight,
is the weight,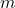 is the mass,
is the mass,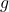 is the acceleration due to gravity (approximately
is the acceleration due to gravity (approximately 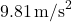 on Earth).
on Earth).
Illustrative Explanation: Consider an astronaut in space. While their mass remains the same (e.g., 80 kg), their weight is effectively zero because the gravitational force is negligible in that environment. Conversely, on Earth, the same astronaut would weigh approximately:
![]()
This illustrates how weight varies with the gravitational field, while mass remains constant.
Role of Mass in Physics
Mass plays a critical role in various physical phenomena and principles:
1. Newton’s Laws of Motion: Mass is central to Newton’s laws, particularly the second law, which relates force, mass, and acceleration. The greater the mass of an object, the more force is required to achieve a given acceleration.
Illustrative Explanation: If you want to push a heavy box across the floor, you need to exert more force than if you were pushing a lighter box. This relationship highlights the importance of mass in determining how objects respond to forces.
2. Conservation of Mass: In chemical reactions, the law of conservation of mass states that mass is neither created nor destroyed. The total mass of reactants equals the total mass of products.
Illustrative Explanation: When burning a piece of wood, the mass of the wood and oxygen consumed equals the mass of the ash, carbon dioxide, and water produced. This principle is fundamental in chemistry and helps balance chemical equations.
3. Gravitational Interactions: Mass is a key factor in gravitational interactions. The more massive an object, the stronger its gravitational pull. This principle governs the motion of celestial bodies and the formation of structures in the universe.
Illustrative Explanation: The Earth, being more massive than the Moon, exerts a stronger gravitational force, which is why the Moon orbits the Earth rather than the other way around.
4. Relativity: In Einstein’s theory of relativity, mass is related to energy through the famous equation ![]() , which states that mass can be converted into energy and vice versa. This relationship has profound implications in nuclear physics and cosmology.
, which states that mass can be converted into energy and vice versa. This relationship has profound implications in nuclear physics and cosmology.
Illustrative Explanation: In nuclear reactions, a small amount of mass is converted into a large amount of energy, as seen in the processes that power the Sun and nuclear reactors.
Applications of Mass in Various Scientific Fields
1. Chemistry: Mass is crucial in stoichiometry, where it is used to calculate the amounts of reactants and products in chemical reactions. Understanding the mass of substances allows chemists to predict reaction outcomes and yields.
Illustrative Explanation: When preparing a solution, a chemist might need to weigh out a specific mass of solute to achieve the desired concentration.
2. Engineering: In engineering, mass is a critical factor in designing structures, vehicles, and machinery. Engineers must consider mass when calculating loads, stresses, and material properties to ensure safety and functionality.
Illustrative Explanation: When designing a bridge, engineers must account for the mass of vehicles that will travel over it, ensuring that the structure can support the weight without collapsing.
3. Astronomy: In astronomy, mass is essential for understanding the dynamics of celestial bodies, such as planets, stars, and galaxies. The mass of an object influences its gravitational interactions and orbital mechanics.
Illustrative Explanation: The mass of a planet determines its gravitational pull, affecting the orbits of its moons and the trajectories of spacecraft that approach it.
4. Medicine: In medicine, mass is used to calculate dosages of medications based on a patient’s body weight. Accurate dosing is critical for ensuring the effectiveness and safety of treatments.
Illustrative Explanation: A doctor may prescribe a medication dosage of 5 mg per kilogram of body weight. For a patient weighing 70 kg, the total dosage would be:
![]()
Conclusion
In conclusion, mass is a fundamental property of matter that plays a vital role in various scientific disciplines. It is a measure of the amount of matter in an object and is essential for understanding physical phenomena, from the motion of objects to chemical reactions and gravitational interactions. By exploring the definition, types, units of measurement, distinction between mass and weight, the role of mass in physics, and its applications in various fields, we gain a deeper appreciation for this critical concept. As we continue to explore the universe and the laws that govern it, the understanding of mass remains a cornerstone of scientific inquiry, influencing our comprehension of the natural world and the principles that underlie it.