Potassium chlorate (KClO₃) is an important chemical compound with a variety of applications in industries ranging from agriculture to pyrotechnics. It is a white crystalline solid that is highly soluble in water and is known for its strong oxidizing properties. This article will provide a detailed exploration of potassium chlorate, including its chemical properties, production methods, uses, safety considerations, and illustrative explanations to enhance understanding.
1. What is Potassium Chlorate?
Potassium chlorate is an inorganic compound composed of potassium (K), chlorine (Cl), and oxygen (O). Its chemical formula is KClO₃, and it is classified as a chlorate, which is a group of compounds containing the chlorate ion (ClO₃⁻). Potassium chlorate is known for its ability to release oxygen when heated or when it reacts with certain substances, making it a powerful oxidizing agent.
Chemical Structure
The structure of potassium chlorate can be represented as follows:
![]()
Where:
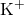 = potassium ion
= potassium ion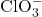 = chlorate ion
= chlorate ion
Illustrative Explanation: Imagine potassium chlorate as a team of superheroes. The potassium ion (K⁺) is like a strong, supportive hero, while the chlorate ion (ClO₃⁻) is a dynamic hero with the ability to release energy (oxygen) when needed. Together, they form a powerful duo capable of performing various tasks (chemical reactions).
2. Properties of Potassium Chlorate
Potassium chlorate possesses several key properties that make it useful in various applications:
A. Physical Properties
- Appearance: KClO₃ is typically found as a white crystalline solid or powder.
- Solubility: It is highly soluble in water, with a solubility of approximately 7.5 g per 100 mL at 20°C.
- Melting Point: The melting point of potassium chlorate is around 356°C (673°F).
Illustrative Explanation: Think of potassium chlorate as a snowflake. Just as a snowflake is delicate and beautiful, KClO₃ appears as a fine white crystal. Its ability to dissolve in water is like a snowflake melting into a puddle, showcasing its solubility.
B. Chemical Properties
- Oxidizing Agent: Potassium chlorate is a strong oxidizer, meaning it can readily donate oxygen to other substances during chemical reactions. This property makes it useful in various applications, including combustion and pyrotechnics.
- Decomposition: When heated, potassium chlorate decomposes to produce potassium chloride (KCl), oxygen gas (O₂), and heat:
![]()
Illustrative Explanation: Imagine potassium chlorate as a firework. When ignited (heated), it releases a burst of energy (oxygen) and transforms into different products (potassium chloride), just as a firework explodes into colorful sparks.
3. Production of Potassium Chlorate
Potassium chlorate can be produced through several methods, with the most common being the reaction of potassium chloride (KCl) with sodium chlorate (NaClO₃) or through the electrolysis of potassium chloride solutions.
A. Reaction with Sodium Chlorate
One method of producing potassium chlorate involves the reaction of potassium chloride with sodium chlorate:
![]()
In this reaction, potassium chloride reacts with sodium chlorate to produce potassium chlorate and sodium chloride (table salt) as a byproduct.
Illustrative Explanation: Think of this reaction as a trade between two friends. One friend (potassium chloride) gives away a toy (chlorate ion) in exchange for a different toy (potassium chlorate), while the other friend (sodium chloride) receives a new toy (sodium chloride) in return.
B. Electrolysis of Potassium Chloride
Another method involves the electrolysis of a potassium chloride solution, where an electric current is passed through the solution, leading to the formation of potassium chlorate.
Illustrative Explanation: Imagine electrolysis as a relay race. The potassium chloride solution is the starting line, and as the electric current (the baton) is passed through, the potassium ions and chloride ions race to their respective electrodes, ultimately forming potassium chlorate as the winning product.
4. Uses of Potassium Chlorate
Potassium chlorate has a wide range of applications across various industries:
A. Agriculture
In agriculture, potassium chlorate is used as a herbicide and a plant growth regulator. It helps control unwanted vegetation and promotes the growth of desired crops.
- Illustrative Explanation: Think of potassium chlorate as a gardener. Just as a gardener removes weeds to allow flowers to flourish, KClO₃ helps eliminate unwanted plants, ensuring that crops can grow strong and healthy.
B. Pyrotechnics
Potassium chlorate is widely used in the production of fireworks, explosives, and safety matches. Its strong oxidizing properties make it an essential component in creating the desired effects in pyrotechnic formulations.
- Illustrative Explanation: Imagine potassium chlorate as the secret ingredient in a recipe for fireworks. Just as a chef carefully selects ingredients to create a delicious dish, pyrotechnicians use KClO₃ to ensure that their fireworks produce stunning displays in the sky.
C. Chemical Synthesis
In the chemical industry, potassium chlorate is used as a reagent in various chemical reactions, including the production of chlorine dioxide (ClO₂) and other chlorinated compounds.
- Illustrative Explanation: Think of potassium chlorate as a versatile tool in a workshop. Just as a multi-tool can be used for various tasks, KClO₃ serves multiple purposes in chemical manufacturing and synthesis.
D. Laboratory Applications
Potassium chlorate is used in laboratories for various analytical and experimental purposes, including the preparation of oxygen gas and as a reagent in redox reactions.
- Illustrative Explanation: Imagine a scientist in a lab conducting experiments. Potassium chlorate is like a trusty assistant, helping the scientist perform various tasks, from generating oxygen to facilitating chemical reactions.
5. Safety Considerations
While potassium chlorate is a valuable compound, it is also a strong oxidizer and can pose safety risks if not handled properly. Some safety considerations include:
A. Oxidizing Nature
KClO₃ can react violently with combustible materials, leading to fire or explosion hazards. It is essential to store potassium chlorate away from flammable substances and to handle it with care.
- Illustrative Explanation: Think of potassium chlorate as a powerful firework. Just as you would handle fireworks with caution to prevent accidents, KClO₃ should be treated with respect to avoid dangerous reactions.
B. Health Hazards
Potassium chlorate can cause irritation to the skin, eyes, and respiratory tract. It is important to wear appropriate personal protective equipment (PPE), such as gloves, goggles, and masks, when handling this chemical.
- Illustrative Explanation: Imagine KClO₃ as a spicy pepper. Just as you would wear gloves and goggles when handling hot peppers to avoid irritation, the same precautions should be taken when working with potassium chlorate.
C. Storage and Disposal
Potassium chlorate should be stored in a cool, dry place, away from incompatible materials such as acids and organic compounds. Disposal should be conducted according to local regulations, as KClO₃ can have harmful effects on the environment if not disposed of properly.
- Illustrative Explanation: Think of potassium chlorate as a valuable treasure. Just as you would store a treasure in a safe place and dispose of it responsibly, KClO₃ should be handled with care to prevent accidents and environmental harm.
6. Conclusion
Potassium chlorate is a versatile and essential chemical compound with a wide range of applications across various industries. Its strong oxidizing properties make it valuable in agriculture, pyrotechnics, chemical synthesis, and laboratory applications. Understanding the properties, production methods, uses, and safety considerations of potassium chlorate is crucial for its effective and safe application. As we continue to explore the role of potassium chlorate in our daily lives, we gain a deeper appreciation for its significance in both industrial processes and everyday products. Whether it’s in the fireworks that light up our celebrations or the herbicides that help our crops thrive, potassium chlorate remains a vital component in the chemistry of life.