Density and volume are fundamental concepts in physics and chemistry that describe the properties of matter. Understanding the relationship between these two concepts is crucial for various scientific applications, including material science, engineering, and environmental science. This article aims to provide an exhaustive overview of the relationship between density and volume, detailing their definitions, mathematical relationships, and practical implications, along with illustrative explanations of each concept.
Understanding Density and Volume
1. Definition of Density
Density is defined as the mass of an object per unit volume. It is a measure of how much matter is packed into a given space. The formula for density (![]() ) is expressed as:
) is expressed as:
![]()
Where:
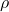 is the density,
is the density,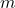 is the mass of the object,
is the mass of the object,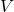 is the volume of the object.
is the volume of the object.
Density is typically expressed in units such as kilograms per cubic meter (kg/m³) or grams per cubic centimeter (g/cm³).
- Illustrative Explanation: Imagine a sponge and a rock of the same size. The sponge is light and fluffy, while the rock is heavy and solid. If you were to weigh both objects, you would find that the rock has a much greater mass than the sponge, even though they occupy the same volume. This difference in mass relative to volume illustrates the concept of density.
2. Definition of Volume
Volume is the amount of space that an object occupies. It is a three-dimensional measurement and can be calculated for various shapes using different formulas. For example, the volume of a cube is calculated as:
![]()
Where ![]() is the length of one side of the cube. For a cylinder, the volume is calculated as:
is the length of one side of the cube. For a cylinder, the volume is calculated as:
![]()
Where ![]() is the radius and
is the radius and ![]() is the height.
is the height.
- Illustrative Explanation: Think of a container filled with water. The volume of water in the container represents the space that the water occupies. If you were to pour the water into a different container, the volume would remain the same, but the shape of the container would change. This illustrates how volume is a measure of space, independent of the object’s shape.
The Relationship Between Density and Volume
3. Mathematical Relationship
The relationship between density and volume can be understood through the density formula mentioned earlier. Rearranging the formula allows us to express volume in terms of mass and density:
![]()
This equation shows that volume is inversely proportional to density when mass is held constant. This means that as the density of a substance increases, its volume decreases, provided the mass remains unchanged.
- Illustrative Explanation: Imagine a balloon filled with air. If you were to compress the balloon, the air inside would become denser, and the volume of the balloon would decrease. Conversely, if you were to let the air out, the density would decrease, and the volume would increase. This illustrates the inverse relationship between density and volume.
4. Practical Implications of Density and Volume Relationship
Understanding the relationship between density and volume has several practical implications in various fields:
a. Material Selection in Engineering
In engineering, selecting materials with the appropriate density is crucial for designing structures and components. Engineers must consider both the weight and volume of materials to ensure safety and efficiency.
- Illustrative Explanation: Consider the construction of a bridge. Engineers must choose materials that provide the necessary strength while minimizing weight. If they select a dense material, such as steel, they must account for the volume it will occupy to ensure the bridge can support the intended loads without being overly heavy.
b. Buoyancy and Floating Objects
The relationship between density and volume is essential for understanding buoyancy. An object will float in a fluid if its density is less than that of the fluid. Conversely, it will sink if its density is greater.
- Illustrative Explanation: Imagine a wooden block and a metal block of the same size. The wooden block has a lower density than water, so it floats, while the metal block has a higher density and sinks. This principle is crucial for designing ships and submarines, where understanding the density of materials and the volume of displaced water determines whether the vessel will float or sink.
c. Density in Chemistry
In chemistry, the relationship between density and volume is vital for calculating concentrations and preparing solutions. Chemists often need to know the density of a substance to determine how much of it to use in a reaction.
- Illustrative Explanation: Suppose a chemist needs to prepare a solution with a specific concentration. By knowing the density of the solute, they can calculate the volume needed to achieve the desired mass. For example, if the density of a chemical is 1.5 g/cm³, and the chemist needs 30 grams, they can use the formula
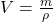 to find that they need 20 cm³ of the chemical.
to find that they need 20 cm³ of the chemical.
Factors Affecting Density and Volume
5. Temperature and Pressure Effects
Both density and volume can be affected by changes in temperature and pressure. Generally, as temperature increases, the volume of a substance expands, leading to a decrease in density. Conversely, increasing pressure can compress a substance, reducing its volume and increasing its density.
- Illustrative Explanation: Consider a sealed container of gas. If you heat the container, the gas molecules gain energy and move faster, causing the gas to expand and occupy a larger volume. As a result, the density of the gas decreases. Conversely, if you compress the gas by applying pressure, its volume decreases, and its density increases.
6. Phase Changes
The phase of a substance (solid, liquid, or gas) also influences its density and volume. Generally, solids have higher densities than liquids, and liquids have higher densities than gases. This is due to the arrangement of particles in different phases.
- Illustrative Explanation: Imagine ice, water, and steam. Ice (solid) has a specific structure that keeps its molecules closely packed, resulting in a higher density than water (liquid), which has more space between molecules. Steam (gas) has even more space between its molecules, leading to a much lower density. This illustrates how phase changes affect both density and volume.
Conclusion
In conclusion, the relationship between density and volume is a fundamental concept that plays a crucial role in various scientific and practical applications. Understanding how density is defined, how it relates to volume, and the factors that influence both properties is essential for fields such as engineering, chemistry, and physics. From material selection in construction to buoyancy in fluids and concentration calculations in chemistry, the interplay between density and volume is vital for understanding the behavior of matter. As we continue to explore the principles of science, the relationship between density and volume will remain a key component of our understanding of the physical world.