The Second Law of Thermodynamics is one of the fundamental principles governing the behavior of energy and heat in physical systems. It provides critical insights into the direction of natural processes, the efficiency of energy conversion, and the concept of entropy. This law has profound implications across various scientific disciplines, including physics, chemistry, engineering, and even cosmology. Understanding the Second Law is essential for grasping the limitations of energy systems, the nature of irreversible processes, and the fundamental principles that govern the universe. This article aims to provide an exhaustive overview of the Second Law of Thermodynamics, including its definitions, implications, applications, and its significance in both theoretical and practical contexts.
Definition of the Second Law of Thermodynamics
The Second Law of Thermodynamics can be articulated in several ways, each emphasizing different aspects of energy transformation and entropy. The most common formulations include:
1. Entropy Increase: In any isolated system, the total entropy—a measure of disorder or randomness—will tend to increase over time. This implies that natural processes tend to move towards a state of greater disorder.
2. Heat Transfer: Heat cannot spontaneously flow from a colder body to a hotter body without external work being performed. This means that energy naturally disperses and spreads out unless constrained by external forces.
3. Efficiency of Heat Engines: No heat engine can be 100% efficient. When converting heat energy into work, some energy is always lost as waste heat to the surroundings. This is often expressed in the context of Carnot’s theorem, which states that the maximum efficiency of a heat engine operating between two heat reservoirs is determined by the temperatures of those reservoirs.
Mathematically, the Second Law can be expressed in terms of entropy (![]() ):
):
![]()
Where:
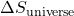 is the change in entropy of the universe,
is the change in entropy of the universe,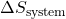 is the change in entropy of the system,
is the change in entropy of the system,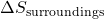 is the change in entropy of the surroundings.
is the change in entropy of the surroundings.
Understanding Entropy
Entropy is a central concept in the Second Law of Thermodynamics. It quantifies the degree of disorder in a system and provides a measure of the energy that is unavailable to do work. Key points about entropy include:
1. Statistical Interpretation: Entropy can be understood statistically as a measure of the number of microscopic configurations that correspond to a macroscopic state. The more ways a system can be arranged while maintaining the same energy, the higher its entropy.
2. Irreversibility: The increase of entropy is associated with the irreversibility of natural processes. For example, when ice melts in a warm environment, the process is irreversible under normal conditions, as the entropy of the system increases.
3. Equilibrium: Systems tend to evolve towards thermodynamic equilibrium, where entropy is maximized. At equilibrium, there are no net macroscopic changes occurring, and the system is in its most probable state.
Implications of the Second Law of Thermodynamics
The Second Law of Thermodynamics has far-reaching implications across various fields:
1. Heat Engines: The Second Law sets fundamental limits on the efficiency of heat engines. The Carnot efficiency, which is the theoretical maximum efficiency of a heat engine operating between two heat reservoirs, is given by:
![]()
Where:
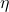 is the efficiency,
is the efficiency,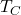 is the absolute temperature of the cold reservoir,
is the absolute temperature of the cold reservoir,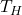 is the absolute temperature of the hot reservoir.
is the absolute temperature of the hot reservoir.
This equation illustrates that no engine can achieve 100% efficiency, as some energy will always be lost as waste heat.
2. Refrigeration and Heat Pumps: The Second Law also governs the operation of refrigerators and heat pumps. These devices transfer heat from a colder region to a hotter region, but they require work input to do so. The coefficient of performance (COP) of a refrigerator is defined as:
![]()
Where:
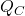 is the heat removed from the cold reservoir,
is the heat removed from the cold reservoir,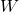 is the work input.
is the work input.
3. Biological Systems: The Second Law has implications in biology, particularly in understanding metabolic processes. Living organisms maintain order and low entropy by consuming energy (from food or sunlight) and expelling waste heat, thus increasing the entropy of their surroundings.
4. Cosmology: The Second Law of Thermodynamics has implications for the fate of the universe. As the universe evolves, it is expected to reach a state of maximum entropy, often referred to as “heat death,” where all energy is uniformly distributed, and no work can be extracted.
Applications of the Second Law of Thermodynamics
The Second Law of Thermodynamics is applied in various practical contexts:
1. Engineering: Engineers use the Second Law to design more efficient engines, refrigerators, and heat exchangers. Understanding the limitations imposed by the Second Law helps in optimizing energy systems and reducing waste.
2. Chemical Reactions: In chemistry, the Second Law is used to predict the spontaneity of reactions. A reaction is spontaneous if it leads to an increase in the total entropy of the system and its surroundings.
3. Environmental Science: The Second Law is relevant in environmental science, particularly in understanding energy flows in ecosystems and the impact of human activities on energy consumption and waste generation.
4. Information Theory: The concept of entropy has been adapted in information theory to quantify the amount of uncertainty or information content in a message. This parallels the thermodynamic definition of entropy, highlighting the interconnectedness of these fields.
Limitations and Misconceptions
While the Second Law of Thermodynamics is a powerful principle, it is often misunderstood. Some common misconceptions include:
1. Perpetual Motion Machines: The Second Law prohibits the existence of perpetual motion machines of the second kind, which would violate the principle of increasing entropy by converting heat entirely into work without any waste.
2. Local Decrease in Entropy: It is possible for entropy to decrease locally in a system (e.g., the formation of ice from water), but this is always accompanied by a greater increase in entropy in the surroundings, ensuring that the total entropy of the universe still increases.
3. Misinterpretation of Efficiency: The Second Law does not imply that all processes are inefficient; rather, it sets limits on the maximum efficiency achievable in energy conversion processes.
Conclusion
The Second Law of Thermodynamics is a fundamental principle that governs the behavior of energy and heat in physical systems. Its implications extend across various scientific disciplines, influencing our understanding of natural processes, energy conversion, and the limitations of technology. By emphasizing the concept of entropy and the directionality of processes, the Second Law provides critical insights into the nature of the universe and the constraints on energy systems. As we continue to explore the implications of the Second Law, it remains a cornerstone of thermodynamics, shaping our understanding of energy, efficiency, and the fundamental principles that govern the physical world. The ongoing study of thermodynamics and its laws will continue to drive advancements in science and technology, ultimately contributing to a more sustainable and efficient future.