Electron configuration refers to the distribution of electrons in an atom’s orbitals, which is fundamental to understanding the chemical behavior and properties of elements. The arrangement of electrons in an atom determines how it interacts with other atoms, influences its reactivity, and dictates its role in various chemical processes. Below, we will explore the significance of electron configuration in detail, highlighting its implications in chemistry, physics, and various applications.
What is Electron Configuration?
Definition
Electron configuration refers to the arrangement of electrons in the atomic orbitals of an atom. It provides a detailed description of how electrons are distributed among the various energy levels and sublevels, which ultimately influences the chemical behavior of the element.
- Illustrative Explanation: Imagine a multi-story building where each floor represents an energy level, and each room on a floor represents an orbital. Just as people occupy different rooms on different floors, electrons occupy various orbitals at different energy levels within an atom.
Key Concepts
1. Energy Levels: Electrons are arranged in energy levels (or shells) around the nucleus of an atom. These levels are designated by principal quantum numbers (n), where n = 1, 2, 3, etc. The higher the value of n, the further the energy level is from the nucleus.
2. Sublevels and Orbitals: Each energy level contains sublevels (s, p, d, f) that have different shapes and capacities for holding electrons. Each sublevel consists of one or more orbitals, which are regions in space where electrons are likely to be found.
3. Pauli Exclusion Principle: This principle states that no two electrons in an atom can have the same set of four quantum numbers. In simpler terms, an orbital can hold a maximum of two electrons, and they must have opposite spins.
4. Hund’s Rule: When electrons occupy orbitals of the same energy (degenerate orbitals), they will fill each orbital singly before pairing up. This minimizes electron-electron repulsion and leads to a more stable configuration.
5. Aufbau Principle: This principle states that electrons fill orbitals starting from the lowest energy level to the highest. The order of filling is determined by the relative energies of the orbitals.
The Electron Configuration Notation
Electron configurations are typically written using a notation that indicates the energy levels, sublevels, and the number of electrons in each sublevel. The general format is:
![]()
For example, the electron configuration of carbon (atomic number 6) is written as:
![]()
This notation indicates that carbon has:
- 2 electrons in the 1s sublevel
- 2 electrons in the 2s sublevel
- 2 electrons in the 2p sublevel
Illustrative Explanation
Think of a library where books are organized by sections (energy levels) and shelves (sublevels). Each shelf can hold a certain number of books (electrons). The electron configuration tells you how many books are on each shelf, helping you understand the organization of the library (the atom).
Steps to Determine Electron Configuration
To determine the electron configuration of an element, follow these steps:
1. Identify the Atomic Number
The atomic number of an element indicates the number of protons in its nucleus, which is equal to the number of electrons in a neutral atom.
- Illustrative Explanation: Imagine counting the number of apples in a basket. Just as the number of apples represents the total quantity, the atomic number represents the total number of electrons in a neutral atom.
2. Use the Aufbau Principle
Start filling the orbitals according to the Aufbau principle, beginning with the lowest energy level and moving to higher levels. Follow the order of filling based on the energy levels and sublevels.
- Illustrative Explanation: Think of filling a glass with water. Just as you pour water into the glass starting from the bottom, you fill the orbitals starting from the lowest energy level.
3. Apply the Pauli Exclusion Principle and Hund’s Rule
Ensure that no orbital contains more than two electrons with opposite spins, and fill degenerate orbitals singly before pairing up.
- Illustrative Explanation: Imagine a game of musical chairs where each chair (orbital) can only hold two players (electrons). Just as players fill chairs one by one before sharing, electrons fill orbitals according to these rules.
4. Write the Configuration
Once all electrons are placed in their respective orbitals, write the electron configuration using the appropriate notation.
- Illustrative Explanation: Consider writing a summary of a story. Just as you summarize the main points, you write the electron configuration to summarize the arrangement of electrons in an atom.
Examples of Electron Configurations
1. Hydrogen (H)
- Atomic Number: 1
- Electron Configuration:
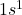
Hydrogen has one electron, which occupies the 1s orbital.
2. Oxygen (O)
- Atomic Number: 8
- Electron Configuration:
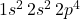
Oxygen has eight electrons, filling the 1s and 2s orbitals and partially filling the 2p orbital.
3. Iron (Fe)
- Atomic Number: 26
- Electron Configuration:
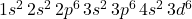
Iron has 26 electrons, filling the 1s, 2s, 2p, 3s, 3p, and 4s orbitals, and partially filling the 3d orbital.
4. Uranium (U)
- Atomic Number: 92
- Electron Configuration:
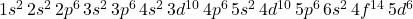
Uranium has 92 electrons, filling the lower energy levels and sublevels before reaching the higher energy levels.
Significance of Electron Configuration
1. Understanding Chemical Properties
a. Reactivity and Bonding
- Valence Electrons: The electron configuration reveals the number of valence electrons in an atom, which are the electrons in the outermost shell. Valence electrons play a crucial role in chemical bonding. For example, elements with similar valence electron configurations tend to exhibit similar chemical properties, leading to the classification of elements into groups in the periodic table.
- Ionic and Covalent Bonds: The electron configuration helps predict whether an atom will form ionic or covalent bonds. Atoms with few valence electrons (like alkali metals) tend to lose electrons and form cations, while atoms with nearly full outer shells (like halogens) tend to gain electrons and form anions. This understanding is essential for predicting the formation of compounds.
b. Periodic Trends
- Trends in the Periodic Table: Electron configuration is key to understanding periodic trends such as electronegativity, ionization energy, and atomic radius. For instance, as you move across a period, the increasing nuclear charge affects the electron configuration, leading to higher ionization energies and smaller atomic radii.
- Group Behavior: Elements in the same group of the periodic table have similar electron configurations in their outer shells, which explains their similar chemical behavior. For example, the noble gases have full outer shells, making them largely inert, while alkali metals have one valence electron, making them highly reactive.
2. Predicting Chemical Reactions
a. Reaction Mechanisms
- Understanding Reactivity: The electron configuration of reactants can help predict the products of chemical reactions. For example, knowing that sodium (Na) has one valence electron and chlorine (Cl) has seven can explain why they react to form sodium chloride (NaCl) through the transfer of electrons.
- Catalysis and Reaction Pathways: Electron configurations can influence the pathways of chemical reactions and the role of catalysts. Certain catalysts work by stabilizing specific electron configurations, thereby lowering the activation energy required for a reaction to proceed.
b. Complex Formation
- Coordination Compounds: In coordination chemistry, the electron configuration of transition metals is crucial for understanding their ability to form complex ions with ligands. The d-orbitals of transition metals can accommodate additional electrons, leading to a variety of oxidation states and coordination geometries.
3. Spectroscopy and Quantum Mechanics
a. Spectroscopic Techniques
- Absorption and Emission Spectra: The electron configuration of an atom determines its energy levels, which in turn affects the wavelengths of light it can absorb or emit. Spectroscopic techniques, such as UV-Vis, IR, and NMR spectroscopy, rely on these transitions to provide information about molecular structure and dynamics.
- Identifying Elements: Electron configurations are fundamental in identifying elements through their characteristic spectral lines. Each element has a unique set of energy levels, leading to distinct spectral fingerprints that can be used in analytical chemistry.
b. Quantum Mechanics
- Wave-Particle Duality: The study of electron configurations is rooted in quantum mechanics, which describes the behavior of electrons as both particles and waves. Understanding electron configurations requires knowledge of quantum numbers, orbitals, and the principles of uncertainty and superposition.
- Pauli Exclusion Principle and Hund’s Rule: The arrangement of electrons in orbitals follows specific rules, such as the Pauli exclusion principle (no two electrons can have the same set of quantum numbers) and Hund’s rule (electrons will occupy degenerate orbitals singly before pairing). These principles are essential for predicting the electron configuration of atoms.
4. Applications in Technology and Industry
a. Material Science
- Semiconductors: The electron configuration of elements like silicon and germanium is critical in the development of semiconductor materials. Understanding how electrons behave in these materials allows for the design of electronic components such as diodes, transistors, and integrated circuits.
- Nanotechnology: In nanomaterials, the electron configuration can influence properties such as conductivity, reactivity, and optical characteristics. Tailoring the electron configuration at the nanoscale can lead to innovative applications in electronics, medicine, and energy storage.
b. Pharmaceuticals
- Drug Design: The electron configuration of atoms in drug molecules affects their interactions with biological targets. Understanding how electron distribution influences molecular shape and reactivity is essential for rational drug design and development.
- Enzyme Activity: Enzymes often rely on specific electron configurations to catalyze biochemical reactions. Understanding the electron configuration of active sites can aid in the design of enzyme inhibitors and activators.
5. Biological Significance
a. Biomolecules
- Protein Structure and Function: The electron configuration of atoms in amino acids influences protein folding and stability. Understanding these configurations is crucial for elucidating the structure-function relationship in proteins.
- DNA and RNA: The electron configuration of nucleotides affects the stability and interactions of nucleic acids. This understanding is vital for studying genetic processes and developing biotechnological applications.
b. Metalloproteins
- Metal Ions in Biology: Many biological processes involve metalloproteins, where metal ions play critical roles in electron transfer and catalysis. The electron configuration of these metal ions determines their reactivity and function in biological systems.
In summary, electron configuration is a fundamental concept in chemistry and physics that has far-reaching implications across various fields. It is essential for understanding chemical properties, predicting reactions, and explaining periodic trends. Additionally, electron configuration plays a critical role in spectroscopy, material science, pharmaceuticals, and biological processes. By providing insights into the behavior of atoms and molecules, electron configuration is a cornerstone of modern scientific understanding and technological advancement. As research continues to evolve, the significance of electron configuration will remain central to the exploration of new materials, chemical reactions, and biological systems.
Conclusion
Electron configuration is a fundamental concept that provides insight into the arrangement of electrons in atoms and their chemical behavior. By understanding the principles, notation, and significance of electron configurations, we can better appreciate the underlying mechanisms that govern chemical reactions and the properties of elements. Whether predicting reactivity, organizing the periodic table, or exploring atomic spectra, electron configuration remains a cornerstone of modern chemistry and physics. As we continue to study and explore this concept, we unlock new possibilities for innovation and discovery in the scientific world.