Specific heat capacity is a fundamental thermodynamic property that describes the amount of heat energy required to change the temperature of a unit mass of a substance by one degree Celsius (or one Kelvin). This property is crucial in various scientific and engineering applications, including thermodynamics, material science, and environmental studies. Understanding specific heat capacity helps in analyzing heat transfer processes, designing thermal systems, and predicting the behavior of materials under varying temperature conditions. This article aims to provide an exhaustive overview of specific heat capacity, including its definition, significance, calculation, types, applications, and illustrative explanations of each concept.
Definition of Specific Heat Capacity
Specific heat capacity (![]() ) is defined as the amount of heat (
) is defined as the amount of heat (![]() ) required to raise the temperature of a unit mass (
) required to raise the temperature of a unit mass (![]() ) of a substance by one degree Celsius (°C) or one Kelvin (K). The mathematical expression for specific heat capacity can be represented as:
) of a substance by one degree Celsius (°C) or one Kelvin (K). The mathematical expression for specific heat capacity can be represented as:
![]()
Where:
 is the specific heat capacity (measured in joules per kilogram per degree Celsius, J/(kg·°C)),
is the specific heat capacity (measured in joules per kilogram per degree Celsius, J/(kg·°C)),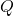 is the heat added or removed (measured in joules, J),
is the heat added or removed (measured in joules, J),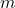 is the mass of the substance (measured in kilograms, kg),
is the mass of the substance (measured in kilograms, kg),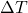 is the change in temperature (measured in degrees Celsius, °C, or Kelvin, K).
is the change in temperature (measured in degrees Celsius, °C, or Kelvin, K).
This equation illustrates that specific heat capacity is a measure of how much energy is needed to change the temperature of a given mass of a substance.
Significance of Specific Heat Capacity
Specific heat capacity is significant for several reasons:
1. Thermal Properties of Materials: Specific heat capacity provides insight into how different materials respond to heat. Materials with high specific heat capacities can absorb and store large amounts of heat without significant temperature changes, making them useful in thermal management applications.
2. Heat Transfer Calculations: In engineering and environmental science, specific heat capacity is essential for calculating heat transfer in various processes, such as heating, cooling, and phase changes. It helps predict how substances will behave when subjected to temperature changes.
3. Energy Efficiency: Understanding specific heat capacity is crucial for designing energy-efficient systems. For example, materials with high specific heat capacities can be used in thermal storage systems to reduce energy consumption.
Calculation of Specific Heat Capacity
To calculate the specific heat capacity of a substance, one must measure the heat added or removed and the corresponding temperature change. The steps involved in the calculation are as follows:
1. Measure the Mass: Determine the mass (![]() ) of the substance in kilograms (kg).
) of the substance in kilograms (kg).
2. Measure the Heat Transfer: Use a calorimeter or other heat transfer measurement devices to determine the amount of heat (![]() ) added or removed from the substance in joules (J).
) added or removed from the substance in joules (J).
3. Measure the Temperature Change: Measure the initial and final temperatures of the substance to calculate the change in temperature (![]() ):
):
![]()
4. Calculate Specific Heat Capacity: Substitute the values of ![]() ,
, ![]() , and
, and ![]() into the specific heat capacity formula:
into the specific heat capacity formula:
![]()
Types of Specific Heat Capacity
Specific heat capacity can be categorized into two main types based on the conditions under which it is measured:
1. Specific Heat Capacity at Constant Pressure (![]() ): This is the specific heat capacity measured when the pressure of the system remains constant. It is particularly relevant for processes occurring in open systems, such as heating water in an open pot. The formula for specific heat capacity at constant pressure is:
): This is the specific heat capacity measured when the pressure of the system remains constant. It is particularly relevant for processes occurring in open systems, such as heating water in an open pot. The formula for specific heat capacity at constant pressure is:
![]()
2. Specific Heat Capacity at Constant Volume (![]() ): This is the specific heat capacity measured when the volume of the system remains constant. It is relevant for processes occurring in closed systems, such as heating a gas in a rigid container. The formula for specific heat capacity at constant volume is:
): This is the specific heat capacity measured when the volume of the system remains constant. It is relevant for processes occurring in closed systems, such as heating a gas in a rigid container. The formula for specific heat capacity at constant volume is:
![]()
The relationship between ![]() and
and ![]() for ideal gases is given by the equation:
for ideal gases is given by the equation:
![]()
Where ![]() is the ideal gas constant.
is the ideal gas constant.
Factors Affecting Specific Heat Capacity
Several factors influence the specific heat capacity of a substance:
1. Material Composition: Different materials have different atomic and molecular structures, which affect their ability to store thermal energy. For example, metals generally have lower specific heat capacities than water, meaning they heat up and cool down more quickly.
2. Phase of the Substance: The specific heat capacity varies with the phase of the substance (solid, liquid, or gas). For example, water has a high specific heat capacity in its liquid state, while ice (solid) has a lower specific heat capacity.
3. Temperature: The specific heat capacity of some materials can change with temperature. For example, the specific heat capacity of gases typically increases with temperature due to increased molecular motion.
4. Pressure: For gases, specific heat capacity can also be affected by pressure. At higher pressures, the specific heat capacity at constant volume (![]() ) may increase due to the increased energy required to maintain the same volume.
) may increase due to the increased energy required to maintain the same volume.
Applications of Specific Heat Capacity
Specific heat capacity has numerous applications across various fields, including:
1. Thermal Management: In engineering, specific heat capacity is used to design thermal management systems, such as heat exchangers, radiators, and cooling systems. Materials with high specific heat capacities are often used in thermal storage applications to absorb and release heat efficiently.
2. Climate Science: In environmental science, specific heat capacity is crucial for understanding heat transfer in the atmosphere and oceans. It helps model climate systems and predict temperature changes in response to various factors, such as greenhouse gas emissions.
3. Cooking and Food Science: Specific heat capacity is important in cooking, as it influences how quickly food heats up or cools down. Understanding the specific heat capacities of different ingredients can help chefs optimize cooking times and temperatures.
4. Material Science: In material science, specific heat capacity is used to characterize materials and assess their thermal properties. It helps in the selection of materials for applications requiring specific thermal performance.
5. Energy Systems: In energy systems, specific heat capacity is essential for analyzing the performance of thermal power plants, refrigeration systems, and heat pumps. It aids in optimizing energy efficiency and reducing operational costs.
Illustrative Examples
To further illustrate the concept of specific heat capacity, consider the following examples:
1. Water vs. Metal: Water has a specific heat capacity of approximately 4.18 J/(kg·°C), while aluminum has a specific heat capacity of about 0.897 J/(kg·°C). This means that to raise the temperature of 1 kg of water by 1°C, 4.18 joules of energy are required, whereas only 0.897 joules are needed to raise the temperature of 1 kg of aluminum by the same amount. This difference explains why water is often used as a coolant in various applications, as it can absorb a significant amount of heat without a large increase in temperature.
2. Heating Ice vs. Water: When heating ice at 0°C to convert it into water at the same temperature, energy is required to overcome the molecular bonds holding the ice together. The specific heat capacity of ice is about 2.09 J/(kg·°C), while that of water is 4.18 J/(kg·°C). This means that more energy is needed to raise the temperature of water than to melt ice, illustrating the concept of latent heat and the importance of specific heat capacity in phase changes.
3. Cooking Pasta: When boiling pasta in water, the specific heat capacity of water plays a crucial role. The high specific heat capacity of water allows it to maintain a relatively stable temperature while cooking the pasta. This ensures that the pasta cooks evenly and absorbs heat gradually, resulting in a better texture.
Conclusion
Specific heat capacity is a fundamental thermodynamic property that provides valuable insights into the thermal behavior of materials. Its definition, calculation, and significance are essential for understanding heat transfer processes in various scientific and engineering applications. By examining the factors that affect specific heat capacity and its applications across different fields, we can appreciate its importance in thermal management, climate science, cooking, and material science. The illustrative examples further highlight the practical implications of specific heat capacity in everyday life and industrial processes. As research continues to advance, the study of specific heat capacity will remain vital for developing new materials, optimizing energy systems, and addressing challenges in thermal management and environmental sustainability. Understanding specific heat capacity not only enriches our knowledge of thermal dynamics but also contributes to innovations that enhance efficiency and performance across diverse applications.