The standard enthalpy of formation, denoted as ![]() , is a fundamental concept in thermodynamics and chemistry that quantifies the heat change associated with the formation of one mole of a compound from its constituent elements in their standard states. This concept is crucial for understanding chemical reactions, calculating reaction enthalpies, and predicting the feasibility of reactions. This article will delve into the definition, significance, calculation methods, applications, and limitations of standard enthalpy of formation, providing a thorough understanding of this essential concept, complete with illustrative explanations to enhance comprehension.
, is a fundamental concept in thermodynamics and chemistry that quantifies the heat change associated with the formation of one mole of a compound from its constituent elements in their standard states. This concept is crucial for understanding chemical reactions, calculating reaction enthalpies, and predicting the feasibility of reactions. This article will delve into the definition, significance, calculation methods, applications, and limitations of standard enthalpy of formation, providing a thorough understanding of this essential concept, complete with illustrative explanations to enhance comprehension.
Definition of Standard Enthalpy of Formation
The standard enthalpy of formation is defined as the change in enthalpy when one mole of a compound is formed from its elements in their most stable forms at a specified temperature (usually 25°C or 298 K) and standard pressure (1 atm). The standard state of a substance refers to its physical state (solid, liquid, or gas) at these conditions.
Mathematical Representation: The standard enthalpy of formation can be expressed as:
![]()
Where:
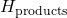 is the enthalpy of the products formed.
is the enthalpy of the products formed.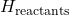 is the enthalpy of the reactants.
is the enthalpy of the reactants.
Illustrative Explanation: Imagine you are baking a cake. The standard enthalpy of formation is like the total energy required to combine all the ingredients (flour, sugar, eggs) in their basic forms (the most stable state) to create one cake (the compound). The energy change during this process represents the standard enthalpy of formation.
Significance of Standard Enthalpy of Formation
The standard enthalpy of formation is significant for several reasons:
1. Thermodynamic Calculations
It allows chemists to calculate the enthalpy changes for chemical reactions using Hess’s Law, which states that the total enthalpy change for a reaction is the sum of the enthalpy changes for individual steps.
Illustrative Explanation: Think of a road trip where you take multiple routes to reach your destination. Regardless of the path you choose, the total distance traveled remains the same. Similarly, the total enthalpy change for a reaction can be calculated by summing the enthalpy changes of individual steps.
2. Predicting Reaction Feasibility
By comparing the standard enthalpy of formation values of reactants and products, chemists can predict whether a reaction is exothermic (releases heat) or endothermic (absorbs heat), which helps in assessing the feasibility of a reaction.
Illustrative Explanation: Imagine a campfire. If you add wood (reactants) and it burns to produce heat and light (products), the reaction is exothermic. Conversely, if you need to add energy (like electricity) to make a chemical reaction occur, it is endothermic. The standard enthalpy of formation helps determine which scenario applies.
3. Standardization
The standard enthalpy of formation provides a standardized reference point for comparing the thermodynamic properties of different substances, facilitating communication and understanding in the scientific community.
Illustrative Explanation: Consider a universal language for measuring distances, like kilometers or miles. Just as these units allow people from different regions to understand distances, the standard enthalpy of formation provides a common reference for comparing energy changes in chemical reactions.
Calculation of Standard Enthalpy of Formation
The standard enthalpy of formation can be calculated using experimental data or derived from tabulated values. The following steps outline the process:
1. Using Hess’s Law
Hess’s Law can be applied to calculate the standard enthalpy of formation by using known enthalpy changes of related reactions. The enthalpy change for the formation of a compound can be determined by manipulating the equations of known reactions.
Illustrative Explanation: Imagine you want to find the total cost of a shopping trip. If you know the prices of individual items (known reactions), you can add them up to find the total cost (enthalpy change for the formation of the compound).
2. Using Standard Enthalpy of Formation Tables
Standard enthalpy of formation values for many compounds are tabulated in reference books. These values can be used directly in calculations to determine the enthalpy change for a reaction.
Illustrative Explanation: Think of a cookbook that lists the nutritional values of various ingredients. If you want to know the total calories in a dish, you can simply look up the values for each ingredient and add them together. Similarly, you can look up the standard enthalpy of formation values for reactants and products to calculate the overall enthalpy change.
3. Experimental Determination
The standard enthalpy of formation can also be determined experimentally using calorimetry, where the heat change during a reaction is measured directly.
Illustrative Explanation: Imagine measuring the temperature change of water when you dissolve salt in it. By carefully monitoring the heat exchange, you can determine the energy change associated with the dissolution process, similar to how calorimetry measures the heat change in chemical reactions.
Applications of Standard Enthalpy of Formation
The standard enthalpy of formation has numerous practical applications across various fields, including:
1. Chemical Engineering
In chemical engineering, the standard enthalpy of formation is used to design and optimize chemical processes, ensuring that reactions are efficient and cost-effective.
Illustrative Explanation: Think of a factory producing chemicals. Engineers use the standard enthalpy of formation to calculate the energy requirements for reactions, helping them design processes that minimize energy consumption and maximize output.
2. Environmental Science
Understanding the standard enthalpy of formation is essential for assessing the environmental impact of chemical reactions, such as combustion processes that release greenhouse gases.
Illustrative Explanation: Imagine a car engine burning fuel. By knowing the standard enthalpy of formation for the fuel and the products of combustion, scientists can evaluate the energy efficiency and environmental impact of the engine, similar to assessing the carbon footprint of a product.
3. Biochemistry
In biochemistry, the standard enthalpy of formation is used to study metabolic pathways and energy changes in biological systems, helping researchers understand how organisms convert energy.
Illustrative Explanation: Picture a plant undergoing photosynthesis. By knowing the standard enthalpy of formation for glucose and oxygen, scientists can analyze the energy changes involved in the process, similar to understanding how a battery stores and releases energy.
4. Material Science
The standard enthalpy of formation is crucial for developing new materials, such as polymers and alloys, by predicting their stability and reactivity.
Illustrative Explanation: Imagine a chef experimenting with new recipes. By understanding the properties of each ingredient (standard enthalpy of formation), the chef can predict how they will react and combine to create a successful dish, just as material scientists predict the behavior of new materials.
Limitations of Standard Enthalpy of Formation
While the standard enthalpy of formation is a valuable concept, there are limitations to consider:
1. Ideal Conditions
The standard enthalpy of formation is based on ideal conditions (25°C and 1 atm), which may not accurately represent real-world scenarios. Deviations from these conditions can affect the enthalpy changes observed in actual reactions.
Illustrative Explanation: Think of a recipe that works perfectly in a controlled kitchen but may not yield the same results in a different environment (like a hot, humid day). Similarly, the standard enthalpy of formation may not account for variations in temperature and pressure.
2. Limited Data Availability
Standard enthalpy of formation values are not available for all compounds, particularly for complex or unstable substances. This limitation can hinder calculations and predictions for certain reactions.
Illustrative Explanation: Imagine trying to bake a cake without knowing the measurements for some ingredients. If the values are missing, it becomes challenging to create the desired dish. Similarly, missing enthalpy values can complicate thermodynamic calculations.
3. Assumptions of Stability
The standard enthalpy of formation assumes that the elements are in their most stable forms. However, some elements can exist in multiple allotropes or forms, which can affect the calculated enthalpy values.
Illustrative Explanation: Consider carbon, which can exist as graphite or diamond. The standard enthalpy of formation assumes a specific form, but if you use a different allotrope, the energy changes may differ, similar to using different types of flour in baking.
Conclusion
In conclusion, the standard enthalpy of formation is a fundamental concept in thermodynamics and chemistry that quantifies the heat change associated with the formation of one mole of a compound from its constituent elements in their standard states. By understanding the definition, significance, calculation methods, applications, and limitations of standard enthalpy of formation, we gain valuable insights into this essential concept. The standard enthalpy of formation plays a critical role in various scientific fields and everyday phenomena, from chemical engineering and environmental science to biochemistry and material science. As we continue to explore the intricacies of thermodynamic principles, we unlock new possibilities for innovation and discovery, ultimately enriching our understanding of the natural world and its complex energy transformations. Through ongoing research and development, the principles governing standard enthalpy of formation will continue to play a vital role in shaping the future of science and technology, contributing to solutions that address global challenges and improve our quality of life.