The Suzuki coupling reaction is a powerful and widely used method in organic chemistry for forming carbon-carbon bonds. Named after the Japanese chemist Akira Suzuki, who was awarded the Nobel Prize in Chemistry in 2010 for his contributions to this field, the reaction involves the coupling of aryl or vinyl boronic acids with aryl or vinyl halides in the presence of a palladium catalyst. This reaction has become a cornerstone in the synthesis of complex organic molecules, including pharmaceuticals, agrochemicals, and advanced materials. This article aims to provide a detailed overview of the Suzuki coupling reaction, including its mechanism, applications, advantages, and illustrative explanations of each concept to enhance understanding.
Overview of the Suzuki Coupling Reaction
What is the Suzuki Coupling Reaction?
The Suzuki coupling reaction is a cross-coupling reaction that allows for the formation of biaryl compounds or alkenes by coupling boronic acids with halogenated aromatic compounds. The general reaction can be represented as follows:
![]()
Where:
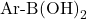 is the boronic acid (or boronate ester).
is the boronic acid (or boronate ester).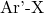 is the aryl or vinyl halide (where X is a halogen such as Cl, Br, or I).
is the aryl or vinyl halide (where X is a halogen such as Cl, Br, or I).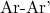 is the resulting biaryl compound.
is the resulting biaryl compound.
Illustrative Explanation
To visualize the Suzuki coupling reaction, imagine two dancers (the reactants) coming together to create a new dance (the product). The boronic acid (one dancer) and the halide (the other dancer) must find the right rhythm (conditions) to perform their dance (the reaction) successfully. With the help of a skilled choreographer (the palladium catalyst), they can create a beautiful new performance (the biaryl compound).
Mechanism of the Suzuki Coupling Reaction
The Suzuki coupling reaction proceeds through a series of well-defined steps, which can be broken down into the following stages:
1. Oxidative Addition
The first step involves the oxidative addition of the aryl or vinyl halide to the palladium catalyst. In this step, the palladium(0) complex reacts with the halide, resulting in the formation of a palladium(II) complex. The halogen atom is replaced by the palladium atom, and the aryl or vinyl group is now bonded to the palladium.
![]()
2. Transmetalation
In the second step, the boronic acid undergoes transmetalation, where the boron atom transfers its aryl group to the palladium complex. This step involves the formation of a new bond between the palladium and the aryl group from the boronic acid.
![]()
3. Reductive Elimination
The final step is reductive elimination, where the palladium complex releases the newly formed biaryl compound and regenerates the palladium(0) catalyst. This step is crucial as it completes the cycle and allows the catalyst to be reused.
![]()
Illustrative Explanation
Think of the mechanism as a relay race. The palladium catalyst is the runner who starts the race (oxidative addition) by grabbing the baton (the halide). Then, the baton is passed to the boronic acid (transmetalation), which runs alongside the palladium. Finally, they cross the finish line together (reductive elimination), resulting in the formation of a new compound while allowing the palladium to run again in the next race.
Applications of the Suzuki Coupling Reaction
The Suzuki coupling reaction has a wide range of applications in various fields, including:
1. Pharmaceutical Synthesis
The Suzuki reaction is extensively used in the synthesis of pharmaceutical compounds. Many drugs contain biaryl structures, and the ability to form these compounds efficiently makes the Suzuki coupling reaction invaluable in drug discovery and development.
2. Material Science
In material science, the Suzuki coupling reaction is employed to synthesize polymers and advanced materials with specific properties. The formation of carbon-carbon bonds allows for the design of materials with tailored functionalities.
3. Agrochemicals
The reaction is also used in the synthesis of agrochemicals, including herbicides and pesticides. The ability to create complex organic molecules is essential for developing effective agricultural products.
4. Organic Electronics
The Suzuki coupling reaction plays a role in the development of organic electronic materials, such as organic light-emitting diodes (OLEDs) and organic photovoltaics (OPVs). The formation of conjugated systems through Suzuki coupling enhances the electronic properties of these materials.
Illustrative Explanation
Imagine the Suzuki coupling reaction as a versatile tool in a craftsman’s workshop. Just as a craftsman uses different tools to create various products, chemists use the Suzuki reaction to build a wide array of compounds in pharmaceuticals, materials, agrochemicals, and electronics. Each application represents a unique project that benefits from the precision and efficiency of this powerful reaction.
Advantages of the Suzuki Coupling Reaction
The Suzuki coupling reaction offers several advantages that contribute to its popularity in organic synthesis:
1. High Selectivity
The reaction is highly selective, allowing for the formation of specific biaryl compounds without significant side reactions. This selectivity is crucial in complex organic syntheses where unwanted byproducts can complicate purification.
2. Tolerance to Functional Groups
The Suzuki reaction is tolerant of various functional groups, making it versatile for use with a wide range of substrates. This tolerance allows chemists to incorporate different functional groups into the final product without affecting the reaction’s efficiency.
3. Mild Reaction Conditions
The Suzuki coupling reaction typically occurs under mild conditions, which minimizes the risk of decomposition of sensitive substrates. This feature is particularly advantageous when working with complex organic molecules.
4. Regeneration of the Catalyst
The ability of the palladium catalyst to be regenerated and reused in multiple cycles enhances the reaction’s efficiency and sustainability. This regeneration reduces the need for excessive amounts of catalyst, making the process more economical.
Illustrative Explanation
Think of the Suzuki coupling reaction as a well-oiled machine. Each component (selectivity, functional group tolerance, mild conditions, and catalyst regeneration) works together seamlessly to produce high-quality products efficiently. Just as a machine operates smoothly with minimal friction, the Suzuki reaction allows chemists to achieve their goals with precision and ease.
Conclusion
In conclusion, the Suzuki coupling reaction is a vital tool in organic chemistry that enables the formation of carbon-carbon bonds through the coupling of boronic acids and halogenated compounds. Understanding its mechanism, applications, advantages, and illustrative explanations enhances our appreciation of this powerful reaction. As we continue to explore the world of organic synthesis, mastering the concepts surrounding the Suzuki coupling reaction will empower chemists to create complex molecules that are essential for pharmaceuticals, materials, agrochemicals, and advanced technologies. The versatility and efficiency of the Suzuki coupling reaction make it a cornerstone of modern organic chemistry, paving the way for innovative discoveries and applications in various fields.