The tetravalency of carbon is a fundamental concept in organic chemistry that explains the unique bonding behavior of carbon atoms. This property is crucial for understanding the structure and reactivity of organic compounds, which form the basis of life on Earth. In this article, we will delve into the definition of tetravalency, the underlying principles of carbon’s bonding, its implications in organic chemistry, and illustrative explanations to enhance understanding.
1. Definition of Tetravalency
Definition: Tetravalency refers to the ability of a carbon atom to form four covalent bonds with other atoms. This property arises from the electronic configuration of carbon, which has four valence electrons in its outer shell. The term “tetravalent” comes from the prefix “tetra,” meaning four, and “valent,” which refers to the bonding capacity of an atom.
Illustrative Explanation: Imagine carbon as a four-legged table. Each leg represents a covalent bond that the carbon atom can form with other atoms. Just as a table needs all four legs to stand firmly, carbon requires four bonds to achieve stability. This tetravalent nature allows carbon to connect with various elements, including hydrogen, oxygen, nitrogen, and other carbon atoms, forming a vast array of organic compounds.
2. Electronic Configuration of Carbon
To understand tetravalency, it is essential to examine the electronic configuration of carbon:
- Atomic Number: Carbon has an atomic number of 6, meaning it has 6 electrons.
- Electron Configuration: The electron configuration of carbon is
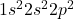 . This configuration shows that carbon has four electrons in its outer shell (2 in the 2s subshell and 2 in the 2p subshell).
. This configuration shows that carbon has four electrons in its outer shell (2 in the 2s subshell and 2 in the 2p subshell).
Illustrative Explanation: Picture carbon as a house with two floors. The first floor (1s) is fully occupied with two electrons (like two rooms filled with furniture), while the second floor (2s and 2p) has four rooms available for guests (valence electrons). To make the house more stable and functional, carbon invites four guests (other atoms) to occupy these rooms, forming bonds and creating a stable structure.
3. Covalent Bonding and Hybridization
The tetravalency of carbon is primarily due to its ability to form covalent bonds through hybridization. Hybridization is the process by which atomic orbitals mix to form new hybrid orbitals that can accommodate bonding.
- Types of Hybridization:
– sp³ Hybridization: In this case, one s orbital and three p orbitals combine to form four equivalent sp³ hybrid orbitals. This arrangement leads to a tetrahedral geometry with bond angles of approximately 109.5°.
– sp² Hybridization: Here, one s orbital and two p orbitals mix to form three sp² hybrid orbitals, resulting in a trigonal planar geometry with bond angles of approximately 120°.
– sp Hybridization: In this scenario, one s orbital and one p orbital combine to form two sp hybrid orbitals, leading to a linear geometry with bond angles of 180°.
Illustrative Explanation: Think of hybridization as a dance party where carbon is the host. In sp³ hybridization, carbon invites four friends (other atoms) to dance in a tetrahedral formation, ensuring everyone has enough space to move (109.5° apart). In sp² hybridization, three friends join in a triangular formation (120° apart), while in sp hybridization, two friends line up in a straight line (180° apart). This flexibility in bonding arrangements allows carbon to form diverse structures.
4. Implications of Tetravalency in Organic Chemistry
The tetravalency of carbon has profound implications for the structure and reactivity of organic compounds:
- Diversity of Organic Compounds: Carbon’s ability to form four bonds enables the creation of a vast array of organic molecules, including hydrocarbons, alcohols, acids, and more. This diversity is the foundation of organic chemistry and biochemistry.
- Isomerism: The tetravalent nature of carbon allows for the existence of isomers—molecules with the same molecular formula but different structural arrangements. For example, butane (C₄H₁₀) can exist as two isomers: n-butane and isobutane.
- Functional Groups: Carbon’s tetravalency allows it to form functional groups, which are specific groups of atoms that impart characteristic properties to organic molecules. Examples include hydroxyl (-OH), carboxyl (-COOH), and amino (-NH₂) groups.
Illustrative Explanation: Imagine carbon as a master builder with a toolbox full of tools (bonds). With four tools at its disposal, carbon can construct a wide variety of structures, from simple houses (hydrocarbons) to complex skyscrapers (biomolecules). Each structure can have different designs (isomers) and features (functional groups), showcasing the versatility of carbon in building the molecular world.
5. Examples of Tetravalency in Carbon Compounds
To illustrate the concept of tetravalency, let’s examine a few examples of carbon compounds:
- Methane (CH₄): In methane, one carbon atom forms four single covalent bonds with four hydrogen atoms. This is a classic example of sp³ hybridization, resulting in a tetrahedral shape.
![]()
- Ethylene (C₂H₄): In ethylene, each carbon atom is sp² hybridized, forming three sigma bonds (two with hydrogen and one with the other carbon) and one pi bond between the two carbon atoms. This results in a planar structure with bond angles of approximately 120°.
![]()
- Acetylene (C₂H₂): In acetylene, each carbon atom is sp hybridized, forming two sigma bonds (one with the other carbon and one with hydrogen) and two pi bonds between the carbon atoms. This results in a linear structure with a bond angle of 180°.
![]()
Illustrative Explanation: Think of these carbon compounds as different types of vehicles. Methane is like a compact car (tetrahedral), ethylene is a sports car with a sleek design (trigonal planar), and acetylene is a long, streamlined train (linear). Each vehicle has its own shape and function, showcasing how carbon’s tetravalency allows for diverse molecular structures.
6. Conclusion
In conclusion, the tetravalency of carbon is a fundamental concept that underpins the vast diversity of organic compounds. Carbon’s ability to form four covalent bonds through hybridization allows it to create a wide range of structures, from simple hydrocarbons to complex biomolecules. Understanding tetravalency is essential for grasping the principles of organic chemistry and the behavior of carbon in various chemical reactions. As we continue to explore the intricacies of carbon chemistry, the tetravalent nature of carbon will remain a cornerstone of our understanding of the molecular world and its significance in the chemistry of life