The f-block elements, also known as the inner transition metals, consist of two series of elements: the lanthanides and the actinides. These elements are characterized by the filling of f orbitals, which play a crucial role in their unique chemical and physical properties. Understanding the f-block elements is essential for grasping the complexities of the periodic table and the behavior of transition metals. This article aims to provide an exhaustive overview of the f-block elements, including their definitions, characteristics, trends, applications, and illustrative explanations of each concept to enhance understanding.
Definition of f-Block Elements
1. Basic Definition:
- The f-block elements are those elements in which the f orbitals are being filled. They are located at the bottom of the periodic table and include the lanthanide series (elements 57 to 71, from lanthanum to lutetium) and the actinide series (elements 89 to 103, from actinium to lawrencium).
Illustrative Explanation: Imagine a large library (the periodic table) with different sections (blocks). The f-block is like a special section at the bottom where rare and unique books (elements) are stored. These books are filled with intricate stories (f orbitals) that reveal the unique characteristics of the elements.
2. Lanthanides and Actinides:
- The lanthanides are the 15 elements following lanthanum (La), characterized by their similar properties and the filling of the 4f subshell. The actinides follow actinium (Ac) and are characterized by the filling of the 5f subshell. The actinides include both naturally occurring and synthetic elements.
Illustrative Example: Think of the lanthanides as a group of siblings (elements) who share similar traits (properties) and live in the same neighborhood (4f subshell). The actinides are like a different group of siblings who have their own unique characteristics (5f subshell) and include some who are adopted (synthetic elements).
Characteristics of f-Block Elements
1. Electron Configuration:
- The general electron configuration of f-block elements can be represented as
![Rendered by QuickLaTeX.com [Xe] 4f^n](https://pengayaan.com/blog/wp-content/uploads/2024/12/quicklatex.com-89c939d31d59eac57327a42c8b11e737_l3.png) for lanthanides and
for lanthanides and ![Rendered by QuickLaTeX.com [Rn] 5f^n](https://pengayaan.com/blog/wp-content/uploads/2024/12/quicklatex.com-edddafc8c255ef2d324e2eb3d41e2ed9_l3.png) for actinides, where
for actinides, where 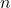 represents the number of electrons in the f subshell. This configuration is crucial for understanding their chemical behavior.
represents the number of electrons in the f subshell. This configuration is crucial for understanding their chemical behavior.
Illustrative Explanation: Imagine a building (atom) with multiple floors (electron shells). The f-block elements have special rooms (f orbitals) on the upper floors that are being filled with furniture (electrons). The arrangement of this furniture determines how the building functions (chemical properties).
2. Variable Oxidation States:
- f-block elements exhibit a wide range of oxidation states due to the involvement of both the f and s orbitals in bonding. This variability allows them to form various compounds and participate in diverse chemical reactions.
Illustrative Example: Picture a chameleon (f-block element) that can change its color (oxidation state) depending on its environment (chemical conditions). This ability to adapt allows the chameleon to blend in and interact with different surroundings (form various compounds).
3. Magnetic Properties:
- Many f-block elements, particularly the lanthanides, exhibit significant magnetic properties due to the presence of unpaired f electrons. This characteristic makes them useful in various applications, including magnets and magnetic materials.
Illustrative Explanation: Imagine a group of tiny magnets (unpaired f electrons) that can align themselves in different directions. When they are all aligned (unpaired electrons), they create a strong magnetic field (magnetic properties), similar to how f-block elements can exhibit magnetism.
4. Colorful Compounds:
- The compounds of f-block elements are often colorful due to the presence of partially filled f orbitals, which allow for electronic transitions that absorb specific wavelengths of light. This property is particularly evident in lanthanide and actinide compounds.
Illustrative Example: Think of a painter (f-block element) mixing different colors (electronic transitions) on a palette. The unique combination of colors creates vibrant hues (colorful compounds) that can be seen in the final artwork (compounds).
Trends in the f-Block Elements
1. Lanthanide Contraction:
- The lanthanide contraction refers to the gradual decrease in ionic and atomic radii of the lanthanide elements as the atomic number increases. This phenomenon occurs due to the poor shielding effect of the f electrons, which leads to a stronger effective nuclear charge.
Illustrative Explanation: Imagine a group of balloons (lanthanide elements) that are being inflated. As more air (electrons) is added, the balloons become slightly smaller (lanthanide contraction) because the surrounding air (nuclear charge) is pushing in on them, making them tighter.
2. Actinide Contraction:
- Similar to the lanthanide contraction, the actinide contraction describes the decrease in size of actinide ions and atoms as the atomic number increases. This contraction is also attributed to the ineffective shielding of the f electrons.
Illustrative Example: Picture a series of boxes (actinide elements) stacked on top of each other. As more boxes are added (increasing atomic number), the boxes become slightly smaller (actinide contraction) because the weight of the boxes above (nuclear charge) compresses them.
3. Stability of Oxidation States:
- The stability of oxidation states varies across the f-block elements. For example, the +3 oxidation state is common for lanthanides, while actinides can exhibit a wider range of oxidation states, including +3, +4, +5, and +6.
Illustrative Explanation: Imagine a seesaw (oxidation states) with different weights (elements) on either side. The lanthanides tend to balance at a specific point (+3 oxidation state), while the actinides can shift their weight to various positions (+3, +4, +5, +6), demonstrating their versatility.
Applications of f-Block Elements
1. Industrial Applications:
- Lanthanides are widely used in various industrial applications, including the production of strong permanent magnets (neodymium), phosphors for color television screens (europium), and catalysts in petroleum refining (cerium).
Illustrative Explanation: Think of a toolbox (industrial applications) filled with specialized tools (lanthanides) that help craftsmen (industries) create unique products. Each tool has a specific purpose, making it essential for the job at hand.
2. Nuclear Applications:
- Actinides, particularly uranium and plutonium, are crucial in nuclear energy production and weaponry. Their ability to undergo fission reactions makes them valuable for generating energy in nuclear reactors.
Illustrative Example: Imagine a powerful engine (nuclear reactor) that runs on a special fuel (actinides). The fuel undergoes a process (fission) that releases a tremendous amount of energy, similar to how actinides are used in nuclear applications.
3. Medical Applications:
- Some f-block elements, such as technetium (though technically a d-block element, it is often associated with f-block elements due to its similar properties), are used in medical imaging and cancer treatment. Radioactive isotopes of certain actinides are also used in targeted alpha therapy.
Illustrative Explanation: Picture a doctor (medical applications) using a special tool (f-block elements) to diagnose and treat patients. The tool helps visualize internal structures (medical imaging) and deliver targeted treatments (cancer therapy), showcasing the importance of f-block elements in medicine.
4. Research and Development:
- The unique properties of f-block elements make them valuable in research and development, particularly in materials science, chemistry, and physics. Their ability to form complex compounds and exhibit unusual magnetic and electronic properties is of great interest to scientists.
Illustrative Explanation: Imagine a laboratory (research and development) where scientists are experimenting with different ingredients (f-block elements) to create new materials. The unique properties of these ingredients allow for innovative discoveries and advancements in technology.
Conclusion
The f-block elements are a fascinating and complex group of elements that play a crucial role in various scientific and industrial applications. By exploring their definitions, characteristics, trends, and applications, we gain valuable insights into the behavior of these unique elements. Just as a skilled conductor leads an orchestra to create a harmonious performance, the interplay of f-block elements orchestrates the interactions of atoms and molecules, allowing us to predict and control their behavior. By mastering these concepts, we equip ourselves with the knowledge to analyze, predict, and influence chemical behavior, enhancing our understanding of chemistry and its applications in various fields. Whether in material science, nuclear energy, or medical research, the principles of f-block elements are integral to the functioning of our world and our daily experiences.