Thermodynamic processes are fundamental transformations that occur in a thermodynamic system, involving changes in energy, heat, and work. These processes are essential for understanding how energy is transferred and transformed in physical systems, and they play a crucial role in various scientific and engineering applications, including engines, refrigerators, and chemical reactions. This article will delve into the definition, types, characteristics, laws governing thermodynamic processes, applications, and limitations, providing a thorough understanding of this essential concept, complete with illustrative explanations to enhance comprehension.
Definition of Thermodynamic Processes
A thermodynamic process is defined as a series of changes that a thermodynamic system undergoes, resulting in a change in its state variables, such as temperature, pressure, volume, and internal energy. These processes can be classified based on how they interact with their surroundings and the nature of the changes that occur within the system.
Illustrative Explanation: Imagine a balloon filled with air. If you heat the balloon, the air inside expands, and the balloon stretches. This change in the balloon’s state (volume, temperature) is an example of a thermodynamic process. The way the balloon interacts with its surroundings (the heat source) also illustrates the concept of thermodynamic processes.
Types of Thermodynamic Processes
Thermodynamic processes can be classified into several categories based on different criteria. The most common classifications include:
1. Isothermal Process
An isothermal process occurs at a constant temperature. During this process, any heat added to the system is used to do work, and the internal energy of the system remains constant. This type of process is often associated with ideal gases.
Mathematical Representation: For an ideal gas undergoing an isothermal process, the relationship between pressure and volume is given by Boyle’s Law:
![]()
Illustrative Explanation: Imagine a sealed syringe filled with gas. If you slowly pull the plunger while keeping the syringe in a water bath at a constant temperature, the gas inside expands (increases in volume) while maintaining the same temperature. The heat from the water bath compensates for the work done by the gas, illustrating an isothermal process.
2. Adiabatic Process
An adiabatic process occurs without any heat exchange between the system and its surroundings. In this process, all the energy changes are due to work done on or by the system. The internal energy of the system changes as a result of work, leading to changes in temperature.
Mathematical Representation: For an ideal gas undergoing an adiabatic process, the relationship between pressure and volume is given by:
![]()
Where ![]() (gamma) is the heat capacity ratio (
(gamma) is the heat capacity ratio (![]() ).
).
Illustrative Explanation: Picture a gas-filled piston that is insulated from its surroundings. If you compress the gas quickly by pushing down on the piston, no heat can escape, and the gas’s temperature rises due to the work done on it. This rapid compression without heat exchange exemplifies an adiabatic process.
3. Isobaric Process
An isobaric process occurs at a constant pressure. During this process, heat is added or removed from the system, resulting in changes in volume and temperature while maintaining constant pressure.
Mathematical Representation: For an isobaric process, the work done on or by the system can be expressed as:
![]()
Where ![]() is the work done,
is the work done, ![]() is the constant pressure, and
is the constant pressure, and ![]() is the change in volume.
is the change in volume.
Illustrative Explanation: Imagine a pot of water being heated on a stove with a lid. As the water heats up, it expands, but the pressure remains constant because the lid allows steam to escape. The water’s temperature increases while the pressure stays the same, illustrating an isobaric process.
4. Isochoric Process
An isochoric process occurs at a constant volume. In this process, any heat added to or removed from the system results in a change in pressure and temperature, but the volume remains unchanged.
Mathematical Representation: For an isochoric process, the change in internal energy can be expressed as:
![]()
Where ![]() is the change in internal energy and
is the change in internal energy and ![]() is the heat added to the system.
is the heat added to the system.
Illustrative Explanation: Consider a sealed, rigid container filled with gas. If you heat the container, the gas molecules gain energy and move faster, increasing the pressure inside the container while the volume remains constant. This scenario exemplifies an isochoric process.
Characteristics of Thermodynamic Processes
Thermodynamic processes exhibit several key characteristics that define their behavior:
1. State Variables
State variables are properties that describe the state of a thermodynamic system, including temperature, pressure, volume, and internal energy. These variables are interrelated, and changes in one can affect the others during a thermodynamic process.
Illustrative Explanation: Think of a car’s dashboard, which displays various indicators such as speed (volume), fuel level (internal energy), and engine temperature (temperature). Changes in one indicator can affect the others, just as state variables interact during thermodynamic processes.
2. Path Dependence
The path taken during a thermodynamic process can affect the work done and heat exchanged. Different processes can lead to different amounts of work and heat transfer, even if the initial and final states are the same.
Illustrative Explanation: Imagine driving from point A to point B. If you take a direct route (isothermal process), it may take less time than if you take a longer, winding road (adiabatic process). Both routes may get you to the same destination, but the journey (path) affects the experience (work and heat transfer).
3. Reversibility
A reversible process is an idealized process that can be reversed without leaving any change in the system or surroundings. In reality, most processes are irreversible due to friction, turbulence, and other factors.
Illustrative Explanation: Picture a perfectly balanced seesaw. If you gently lower one side, it can return to its original position without any loss of energy (reversible). However, if you drop a heavy weight on one side, it will not return to its original position without external intervention (irreversible).
Laws Governing Thermodynamic Processes
Thermodynamic processes are governed by several fundamental laws, including:
1. First Law of Thermodynamics
The First Law of Thermodynamics, also known as the law of energy conservation, states that energy cannot be created or destroyed, only transformed from one form to another. The change in internal energy of a system is equal to the heat added to the system minus the work done by the system:
![]()
Where:
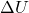 = Change in internal energy
= Change in internal energy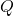 = Heat added to the system
= Heat added to the system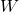 = Work done by the system
= Work done by the system
Illustrative Explanation: Imagine a battery charging a phone. The energy from the battery (heat) is transformed into electrical energy (work) that powers the phone. The total energy remains constant, illustrating the First Law of Thermodynamics.
2. Second Law of Thermodynamics
The Second Law of Thermodynamics states that the total entropy of an isolated system can never decrease over time. In any energy transfer or transformation, the entropy of the universe increases, indicating the direction of spontaneous processes.
Illustrative Explanation: Think of a messy room. Over time, it tends to get messier (increased entropy) unless you actively clean it (decrease entropy). Similarly, natural processes tend to move toward a state of greater disorder.
3. Third Law of Thermodynamics
The Third Law of Thermodynamics states that as the temperature of a system approaches absolute zero, the entropy of a perfect crystal approaches zero. This law establishes an absolute reference point for the determination of entropy.
Illustrative Explanation: Imagine a perfectly organized bookshelf. As you cool the room down to absolute zero, the books (molecules) become perfectly aligned and organized, resulting in minimal disorder (entropy approaching zero).
Applications of Thermodynamic Processes
Thermodynamic processes have numerous practical applications across various fields, including:
1. Heat Engines
Heat engines operate based on thermodynamic processes, converting heat energy into mechanical work. Understanding these processes is essential for designing efficient engines, such as internal combustion engines and steam turbines.
Illustrative Explanation: Picture a car engine. It takes in fuel (heat), converts it into mechanical energy (work), and expels exhaust gases. The thermodynamic processes involved in this transformation are crucial for the engine’s efficiency.
2. Refrigeration and Air Conditioning
Refrigeration and air conditioning systems rely on thermodynamic processes to transfer heat from one location to another. Understanding these processes is essential for designing efficient cooling systems.
Illustrative Explanation: Think of a refrigerator as a heat pump. It removes heat from the inside (cooling) and releases it outside (heating). The thermodynamic processes involved in this transfer are critical for maintaining the desired temperature.
3. Chemical Reactions
Thermodynamic processes play a significant role in chemical reactions, influencing reaction rates, equilibrium, and energy changes. Understanding these processes is essential for optimizing chemical production and developing new materials.
Illustrative Explanation: Imagine baking a cake. The heat (thermodynamic process) causes the ingredients to react and transform into a delicious cake. Understanding the energy changes involved helps bakers achieve the perfect result.
4. Environmental Science
Thermodynamic processes are essential for understanding natural phenomena, such as weather patterns, climate change, and energy transfer in ecosystems. This knowledge is crucial for addressing environmental challenges.
Illustrative Explanation: Picture the water cycle. As water evaporates (thermodynamic process), it transforms into vapor, rises into the atmosphere, and eventually condenses into clouds. Understanding these processes helps scientists predict weather patterns and climate changes.
Limitations of Thermodynamic Processes
While thermodynamic processes are fundamental to understanding energy transformations, there are limitations to consider:
1. Idealizations
Many thermodynamic models assume ideal conditions, such as perfect gases and reversible processes. In reality, these idealizations may not accurately represent real-world systems, leading to discrepancies in predictions.
Illustrative Explanation: Think of a perfect circle drawn on paper. While it represents an ideal shape, real circles (like wheels) may have imperfections. Similarly, idealized thermodynamic models may not account for real-world complexities.
2. Irreversibility
Most natural processes are irreversible, meaning they cannot be reversed without external intervention. This irreversibility complicates the analysis of thermodynamic systems and limits the efficiency of energy transformations.
Illustrative Explanation: Imagine a glass of spilled milk. Once it spills, you cannot simply reverse the process and return the milk to the glass without cleaning up the mess. Similarly, many thermodynamic processes cannot be reversed without external work.
3. Energy Losses
In real systems, energy losses due to friction, heat dissipation, and other factors can reduce the efficiency of thermodynamic processes. Understanding these losses is essential for optimizing system performance.
Illustrative Explanation: Picture a bicycle with flat tires. As you pedal, more energy is lost to friction with the ground, making it harder to ride. Similarly, energy losses in thermodynamic processes can reduce efficiency and performance.
Conclusion
In conclusion, thermodynamic processes are fundamental transformations that describe how energy, heat, and work interact within a thermodynamic system. By understanding the definition, types, characteristics, laws governing thermodynamic processes, applications, and limitations, we gain valuable insights into this essential concept. Thermodynamic processes play a critical role in various scientific fields and everyday phenomena, from engines and refrigeration to chemical reactions and environmental science. As we continue to explore the intricacies of thermodynamic processes, we unlock new possibilities for innovation and discovery, ultimately enriching our understanding of the natural world and its complex energy transformations. Through ongoing research and development, the principles governing thermodynamic processes will continue to play a vital role in shaping the future of science and technology, contributing to solutions that address global challenges and improve our quality of life.