Volumetric analysis, also known as titrimetric analysis, is a quantitative analytical method used to determine the concentration of a substance in a solution by measuring the volume of a titrant required to react with the analyte. This technique is widely employed in chemistry, biochemistry, environmental science, and various industrial applications due to its accuracy, simplicity, and cost-effectiveness. This article will provide a detailed exploration of volumetric analysis, including its principles, types, procedures, calculations, applications, and illustrative explanations to enhance understanding.
1. Principles of Volumetric Analysis
Basic Concept
The fundamental principle of volumetric analysis is based on the stoichiometry of chemical reactions. When a solution of known concentration (the titrant) is added to a solution of unknown concentration (the analyte), a chemical reaction occurs. The point at which the reaction is complete is called the equivalence point, and it can be determined using various indicators or instruments.
Stoichiometry
Stoichiometry is the calculation of reactants and products in chemical reactions. In volumetric analysis, the relationship between the volumes and concentrations of the titrant and analyte is governed by the balanced chemical equation of the reaction.
Illustrative Explanation
Imagine a seesaw (the chemical reaction) where two children (the reactants) are balanced. If one child (the titrant) is heavier (more concentrated), you need to add a certain amount of the lighter child (the analyte) to achieve balance (the equivalence point). The amount of each child represents the volume and concentration of the solutions involved in the reaction.
2. Types of Volumetric Analysis
Volumetric analysis can be categorized into several types based on the nature of the reaction and the method of detection:
1. Acid-Base Titration
Description: This type involves the reaction between an acid and a base. An acid-base indicator is used to determine the endpoint of the titration, which corresponds to the equivalence point.
Example: Titrating hydrochloric acid (HCl) with sodium hydroxide (NaOH) to determine the concentration of the acid.
2. Redox Titration
Description: In redox titrations, the reaction involves the transfer of electrons between the titrant and the analyte. A redox indicator or potentiometric method is used to detect the endpoint.
Example: Titrating potassium permanganate (KMnO₄) against iron(II) sulfate (FeSO₄) to determine the concentration of iron.
3. Precipitation Titration
Description: This type involves the formation of a precipitate during the reaction. The endpoint is determined by visual inspection or using indicators that change color when the precipitate forms.
Example: Titrating silver nitrate (AgNO₃) with sodium chloride (NaCl) to determine the concentration of chloride ions.
4. Complexometric Titration
Description: In complexometric titrations, a complexing agent is used to form a stable complex with the analyte. The endpoint is detected using indicators that change color when the complex is formed or dissociated.
Example: Titrating calcium ions (Ca²⁺) with ethylenediaminetetraacetic acid (EDTA) to determine the concentration of calcium in a sample.
Illustrative Explanation
Think of volumetric analysis as a series of games, each with its own rules. In the acid-base game, players (acids and bases) interact to find balance (neutralization). In the redox game, players exchange tokens (electrons) to achieve victory (reaction completion). In the precipitation game, players build towers (precipitates) until they reach a certain height (endpoint). Each game represents a different type of volumetric analysis, but they all aim to achieve a common goal: determining the concentration of a substance.
3. Procedure of Volumetric Analysis
The procedure for conducting volumetric analysis typically involves the following steps:
1. Preparation of Solutions
- Titrant: Prepare a standard solution of known concentration (the titrant).
- Analyte: Prepare the solution of unknown concentration (the analyte) in a suitable container, such as a flask.
2. Selection of Indicator
Choose an appropriate indicator that will signal the endpoint of the titration. The choice of indicator depends on the type of titration being performed.
3. Titration Process
1. Setup: Fill a burette with the titrant solution and record the initial volume.
2. Titration: Slowly add the titrant to the analyte while continuously swirling the flask to ensure thorough mixing.
3. Endpoint Detection: Observe the color change of the indicator or use a potentiometer to determine the endpoint of the titration.
4. Final Volume Measurement: Record the final volume of the titrant in the burette.
4. Calculations
Calculate the concentration of the analyte using the formula:
![]()
Where:
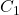 = concentration of the titrant
= concentration of the titrant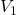 = volume of the titrant used
= volume of the titrant used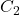 = concentration of the analyte
= concentration of the analyte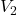 = volume of the analyte
= volume of the analyte
Illustrative Explanation
Imagine a cooking recipe where you need to measure ingredients (solutions) precisely. You start with a known amount of flour (titrant) and gradually add it to a bowl of batter (analyte) while stirring (mixing). When the batter reaches the perfect consistency (endpoint), you stop adding flour and measure how much you used (final volume). You can then calculate how much batter you have based on the amount of flour you added (concentration calculations).
4. Applications of Volumetric Analysis
Volumetric analysis has numerous applications across various fields:
1. Environmental Analysis
Used to determine the concentration of pollutants in water and soil samples, helping to monitor environmental health.
2. Pharmaceutical Industry
Employed in quality control to ensure the correct dosage of active ingredients in medications.
3. Food Industry
Used to analyze the acidity of food products, ensuring compliance with safety standards and quality control.
4. Clinical Laboratories
Applied in medical diagnostics to measure the concentration of substances in blood and urine samples.
Illustrative Explanation
Think of volumetric analysis as a detective tool used in various investigations. In environmental analysis, it helps detectives (scientists) uncover hidden pollutants in water. In the pharmaceutical industry, it ensures that the right amount of medicine is delivered to patients. In the food industry, it checks that food products are safe to eat. In clinical laboratories, it helps diagnose health issues by analyzing body fluids. Each application is like a different case that requires precise measurements to solve.
5. Advantages and Limitations
Advantages
- Accuracy: Volumetric analysis provides precise and reliable results when performed correctly.
- Simplicity: The technique is relatively straightforward and does not require complex instrumentation.
- Cost-Effectiveness: It is an economical method for determining concentrations, especially in routine analyses.
Limitations
- Indicator Limitations: The choice of indicator can affect the accuracy of the endpoint detection.
- Interference: Other substances in the solution may interfere with the reaction, leading to inaccurate results.
- Skill Requirement: Proper technique and experience are necessary to achieve reliable results.
Illustrative Explanation
Think of volumetric analysis as a tool in a toolbox. It has many advantages, like being accurate and easy to use, making it a go-to tool for many tasks. However, just like any tool, it has limitations. If the wrong indicator is chosen (like using a hammer for a screw), it can lead to problems. Additionally, if there are too many distractions (interfering substances), it can make the job harder. Skilled hands (experienced technicians) are needed to wield this tool effectively.
Conclusion
In conclusion, volumetric analysis is a fundamental technique in analytical chemistry that allows for the precise determination of the concentration of substances in solution. By understanding its principles, types, procedures, applications, and advantages and limitations, we can appreciate the significance of this method in various scientific and industrial fields. Whether it is used in environmental monitoring, pharmaceutical quality control, food safety, or clinical diagnostics, volumetric analysis remains an essential tool for chemists and scientists. As technology advances, the methods and applications of volumetric analysis will continue to evolve, further enhancing our ability to analyze and understand the world around us.