Weak bases are a fundamental category of bases that do not completely dissociate into ions in an aqueous solution. Unlike strong bases, which fully ionize and release a high concentration of hydroxide ions (![]() ), weak bases only partially ionize, resulting in a lower concentration of
), weak bases only partially ionize, resulting in a lower concentration of ![]() ions in solution. This article will delve into the definition of weak bases, their chemical properties, examples, the concept of base dissociation constant (
ions in solution. This article will delve into the definition of weak bases, their chemical properties, examples, the concept of base dissociation constant (![]() ), factors affecting their strength, applications, and significance, providing illustrative explanations for each concept.
), factors affecting their strength, applications, and significance, providing illustrative explanations for each concept.
Definition of Weak Bases
A weak base is an inorganic or organic compound that only partially ionizes in solution, meaning that when it is dissolved in water, not all of its molecules release hydroxide ions. This partial ionization results in an equilibrium between the undissociated base and the ions produced.
Illustrative Explanation: Think of a weak base as a reserved person at a gathering. Just as the reserved person may not engage with everyone in the room, a weak base does not fully dissociate into ions. Instead, it maintains a balance between the undissociated base and the ions in solution.
Chemical Properties of Weak Bases
Weak bases exhibit several key chemical properties that distinguish them from strong bases:
1. Partial Ionization: In a solution of a weak base, only a fraction of the base molecules dissociate into hydroxide ions. This means that the concentration of hydroxide ions is lower compared to a strong base of the same concentration.
Illustrative Explanation: Imagine a classroom where only a few students raise their hands to answer questions. Just as only a portion of the students participate, only a fraction of the weak base molecules release hydroxide ions.
2. Equilibrium: The dissociation of weak bases establishes an equilibrium between the undissociated base and the ions produced. This equilibrium can be represented as:
![]()
where ![]() is the weak base,
is the weak base, ![]() is the conjugate acid, and
is the conjugate acid, and ![]() is the hydroxide ion.
is the hydroxide ion.
Illustrative Explanation: Think of a seesaw that balances between two sides. Just as the seesaw remains in equilibrium, the weak base maintains a balance between the undissociated molecules and the ions in solution.
3. pH Levels: Solutions of weak bases have lower pH values compared to strong bases at the same concentration. This is because the concentration of hydroxide ions is lower in weak base solutions.
Illustrative Explanation: Picture a scale measuring basicity. Just as a lighter weight (lower concentration of ![]() ) results in a lower position on the scale, weak bases produce a higher pH due to their partial ionization.
) results in a lower position on the scale, weak bases produce a higher pH due to their partial ionization.
Examples of Weak Bases
Several common weak bases are frequently encountered in chemistry and everyday life:
1. Ammonia (![]() ): Ammonia is a classic example of a weak base. When dissolved in water, it partially ionizes to produce hydroxide ions and ammonium ions (
): Ammonia is a classic example of a weak base. When dissolved in water, it partially ionizes to produce hydroxide ions and ammonium ions (![]() ).
).
Illustrative Explanation: Think of ammonia as a group of friends at a café. Just as not all friends order coffee, not all ammonia molecules release hydroxide ions when dissolved in water.
![]()
2. Sodium Bicarbonate (![]() ): Sodium bicarbonate, commonly known as baking soda, is another example of a weak base. It can react with acids to produce carbon dioxide, water, and a salt.
): Sodium bicarbonate, commonly known as baking soda, is another example of a weak base. It can react with acids to produce carbon dioxide, water, and a salt.
Illustrative Explanation: Imagine sodium bicarbonate as a helper in a kitchen. Just as the helper assists in baking without taking over the entire process, sodium bicarbonate acts as a weak base that can neutralize acids without fully dissociating.
3. Pyridine (![]() ): Pyridine is an organic compound that acts as a weak base. It can accept protons from acids, forming pyridinium ions (
): Pyridine is an organic compound that acts as a weak base. It can accept protons from acids, forming pyridinium ions (![]() ).
).
Illustrative Explanation: Think of pyridine as a student in a classroom. Just as the student can ask questions but doesn’t dominate the discussion, pyridine can accept protons without fully ionizing.
![]()
Base Dissociation Constant (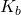 )
)
The strength of a weak base is quantitatively expressed by its base dissociation constant (![]() ). The
). The ![]() value indicates the extent to which a base dissociates in solution. A higher
value indicates the extent to which a base dissociates in solution. A higher ![]() value signifies a stronger weak base, while a lower
value signifies a stronger weak base, while a lower ![]() value indicates a weaker weak base.
value indicates a weaker weak base.
The expression for ![]() is given by:
is given by:
![]()
where ![]() is the concentration of the conjugate acid,
is the concentration of the conjugate acid, ![]() is the concentration of hydroxide ions, and
is the concentration of hydroxide ions, and ![]() is the concentration of the undissociated weak base.
is the concentration of the undissociated weak base.
Illustrative Explanation: Think of ![]() as a popularity rating for weak bases. Just as a higher rating indicates a more popular person, a higher
as a popularity rating for weak bases. Just as a higher rating indicates a more popular person, a higher ![]() value indicates a weak base that dissociates more readily into ions.
value indicates a weak base that dissociates more readily into ions.
Factors Affecting the Strength of Weak Bases
Several factors can influence the strength of weak bases:
1. Molecular Structure: The structure of the base molecule affects its ability to accept protons. For example, the presence of electronegative atoms can stabilize the conjugate acid, making the base stronger.
Illustrative Explanation: Imagine a team of athletes. Just as some athletes have better training and skills, certain molecular structures allow weak bases to accept protons more effectively.
2. Resonance Stabilization: If the conjugate acid of a weak base can stabilize its positive charge through resonance, the base will be stronger. Resonance allows for the delocalization of charge, making it easier for the base to accept a proton.
Illustrative Explanation: Think of resonance as a group of friends sharing a secret. Just as sharing the secret among friends makes it less burdensome, resonance stabilizes the positive charge on the conjugate acid, making it easier for the weak base to accept protons.
3. Solvent Effects: The solvent in which the weak base is dissolved can affect its dissociation. Polar solvents can stabilize ions, enhancing the dissociation of the weak base.
Illustrative Explanation: Picture a swimmer in a pool. Just as the water (solvent) can help the swimmer move more easily, a polar solvent can stabilize ions, facilitating the dissociation of weak bases.
Applications of Weak Bases
Weak bases have numerous applications across various fields:
1. Food Industry: Weak bases like sodium bicarbonate are commonly used in baking and cooking. They help neutralize acids and can act as leavening agents.
Illustrative Explanation: Think of weak bases as the secret ingredients in a recipe. Just as a pinch of baking soda can make a cake rise, weak bases play a crucial role in food preparation.
2. Biological Systems: Weak bases are essential in biological processes. For example, bicarbonate ions help maintain the pH balance in blood and regulate respiration.
Illustrative Explanation: Imagine a thermostat regulating room temperature. Just as the thermostat keeps the environment comfortable, weak bases help maintain pH balance in living organisms.
3. Buffer Solutions: Weak bases are crucial components of buffer solutions, which resist changes in pH when small amounts of acids or bases are added. Buffers are essential in biological and chemical systems to maintain stable pH levels.
Illustrative Explanation: Think of a buffer as a sponge that absorbs excess water. Just as the sponge prevents flooding, buffers help stabilize pH levels in solutions.
4. Chemical Synthesis: Weak bases are often used in chemical reactions and synthesis processes. They can act as catalysts or reactants in various organic reactions.
Illustrative Explanation: Picture a workshop where tools are used to create new products. Just as tools facilitate the construction process, weak bases play a vital role in chemical synthesis.
Significance of Weak Bases
Understanding weak bases is significant for several reasons:
1. Fundamental Chemical Concept: Weak bases are a fundamental concept in chemistry that helps explain the behavior of acids and bases, influencing chemical reactions and equilibria.
Illustrative Explanation: Think of weak bases as the building blocks of acid-base chemistry. Just as foundational knowledge supports advanced concepts, understanding weak bases is essential for grasping more complex chemical phenomena.
2. Educational Value: Weak bases are commonly taught in chemistry courses, providing students with hands-on experience in conducting experiments and observing acid-base behavior.
Illustrative Explanation: Imagine a classroom where students conduct experiments. Just as hands-on learning enhances understanding, studying weak bases helps students grasp key concepts in chemistry.
3. Environmental Impact: Weak bases play a role in environmental processes, such as the natural carbon cycle and the buffering of natural waters. Understanding their behavior is essential for addressing environmental issues.
Illustrative Explanation: Picture a gardener tending to a garden. Just as the gardener nurtures plants for a healthy ecosystem, understanding weak bases helps scientists develop sustainable practices for environmental protection.
4. Research and Development: Weak bases are essential in research and development, enabling scientists to explore new materials, synthesize compounds, and develop innovative technologies.
Illustrative Explanation: Think of researchers as explorers venturing into uncharted territory. Just as explorers seek new discoveries, scientists use weak bases to uncover new materials and advance technology.
Conclusion
In conclusion, weak bases are a vital class of bases characterized by their partial ionization in solution. Their underlying chemical properties, examples, base dissociation constant (![]() ), factors affecting their strength, and practical applications highlight their significance in various fields, including biology, environmental science, and industry. Understanding weak bases not only enhances our knowledge of chemical processes but also emphasizes their role in everyday life and technological advancements. As we continue to explore the complexities of weak bases, we can harness their potential for innovation and improvement in science and society. Through ongoing research and education, we can deepen our understanding of weak bases and their applications, ensuring that we utilize their properties for the benefit of individuals and the environment as a whole.
), factors affecting their strength, and practical applications highlight their significance in various fields, including biology, environmental science, and industry. Understanding weak bases not only enhances our knowledge of chemical processes but also emphasizes their role in everyday life and technological advancements. As we continue to explore the complexities of weak bases, we can harness their potential for innovation and improvement in science and society. Through ongoing research and education, we can deepen our understanding of weak bases and their applications, ensuring that we utilize their properties for the benefit of individuals and the environment as a whole.