In the realm of chemical kinetics, the concept of reaction order is crucial for understanding how the rate of a chemical reaction depends on the concentration of reactants. Among the various types of reaction orders, zero order reactions represent a unique category where the rate of reaction is independent of the concentration of the reactants. This article will delve into the definition of zero order reactions, their characteristics, mathematical representation, examples, and applications, providing illustrative explanations for each concept.
Definition of Zero Order Reaction
A zero order reaction is a type of chemical reaction in which the rate of reaction remains constant and does not change with varying concentrations of the reactants. In other words, the rate of the reaction is zero order with respect to the reactants involved, meaning that the concentration of the reactants does not influence the speed at which the reaction occurs.
Illustrative Explanation: Imagine a car driving on a straight road at a constant speed. No matter how much you press the accelerator (concentration of reactants), the car maintains the same speed (rate of reaction). In a zero order reaction, the concentration of reactants does not affect the rate; it remains constant until the reactants are depleted.
Characteristics of Zero Order Reactions
Zero order reactions exhibit several distinct characteristics:
1. Constant Rate: The rate of a zero order reaction is constant and does not depend on the concentration of the reactants. This means that the reaction proceeds at a uniform speed until the reactants are exhausted.
Illustrative Explanation: Think of a water faucet that is fully open, allowing water to flow out at a steady rate. No matter how much water is in the tank (concentration of reactants), the flow rate remains the same until the tank is empty.
2. Rate Law: The rate law for a zero order reaction can be expressed as:
![]()
where ![]() is the rate constant. This indicates that the rate is independent of the concentration of the reactants.
is the rate constant. This indicates that the rate is independent of the concentration of the reactants.
Illustrative Explanation: Imagine a factory producing widgets at a fixed rate (k). Regardless of how many raw materials (reactants) are available, the factory produces the same number of widgets per hour.
3. Half-Life: The half-life of a zero order reaction is directly proportional to the initial concentration of the reactants. It can be calculated using the formula:
![]()
where ![]() is the initial concentration of the reactant and
is the initial concentration of the reactant and ![]() is the rate constant.
is the rate constant.
Illustrative Explanation: Think of a cake being eaten at a constant rate. If you start with a larger cake (higher initial concentration), it will take longer to eat half of it compared to a smaller cake (lower initial concentration). The time it takes to reach half is dependent on the initial size.
4. Graphical Representation: When plotting the concentration of the reactant versus time for a zero order reaction, the graph will yield a straight line with a negative slope. The slope of this line is equal to ![]() .
.
Illustrative Explanation: Picture a straight downhill slope on a graph. As time passes, the height of the slope (concentration of reactants) decreases uniformly, illustrating the constant rate of reaction.
Mathematical Representation
The mathematical representation of a zero order reaction can be derived from the integrated rate law. For a zero order reaction, the relationship between concentration and time can be expressed as:
![]()
where:
![Rendered by QuickLaTeX.com [A]](https://pengayaan.com/blog/wp-content/uploads/2024/11/quicklatex.com-b2e794eaaca376db17f79ff4fb8089b8_l3.png) is the concentration of the reactant at time
is the concentration of the reactant at time 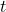 ,
,![Rendered by QuickLaTeX.com [A]_0](https://pengayaan.com/blog/wp-content/uploads/2024/11/quicklatex.com-ab61ba0e45afdbcde627d9bec7f88817_l3.png) is the initial concentration of the reactant,
is the initial concentration of the reactant,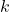 is the rate constant,
is the rate constant,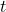 is the time elapsed.
is the time elapsed.
Illustrative Explanation: Imagine a bucket filled with water (initial concentration) that has a hole at the bottom (reaction). As time passes, water drains out at a constant rate (k). The amount of water left in the bucket decreases linearly over time, illustrating the relationship between concentration and time in a zero order reaction.
Examples of Zero Order Reactions
Zero order reactions can be observed in various chemical processes. Here are a few notable examples:
1. Decomposition of Nitrous Oxide: The thermal decomposition of nitrous oxide (N₂O) at high temperatures can be considered a zero order reaction. The rate of decomposition remains constant regardless of the concentration of nitrous oxide.
Illustrative Explanation: Think of a candle burning steadily. No matter how much wax (N₂O) is present, the candle burns at a constant rate until all the wax is consumed.
2. Enzyme-Catalyzed Reactions: In certain enzyme-catalyzed reactions, when the substrate concentration is much higher than the enzyme concentration, the reaction can behave as a zero order reaction. The enzyme becomes saturated, and the rate of reaction remains constant.
Illustrative Explanation: Imagine a busy restaurant where the number of customers (substrate) far exceeds the number of waiters (enzymes). Even if more customers arrive, the waiters can only serve at a fixed rate until they are overwhelmed.
3. Photodecomposition of Organic Compounds: The photodecomposition of certain organic compounds under constant light intensity can also exhibit zero order kinetics, where the rate of decomposition remains constant regardless of the concentration of the compound.
Illustrative Explanation: Picture a plant under a constant light source. No matter how many leaves (organic compounds) are present, the rate of photosynthesis (decomposition) remains steady as long as the light is constant.
Applications of Zero Order Reactions
Understanding zero order reactions has practical implications in various fields:
1. Pharmaceuticals: In drug delivery systems, zero order kinetics can be desirable for maintaining a constant drug concentration in the bloodstream over time. This is particularly important for medications that require steady dosing.
Illustrative Explanation: Think of a slow-release medication as a time-release capsule that dispenses a consistent amount of medicine (drug concentration) into the body over an extended period, ensuring effective treatment.
2. Chemical Manufacturing: In industrial processes, controlling reaction rates is crucial for optimizing production. Zero order reactions can be advantageous in scenarios where a constant output is desired.
Illustrative Explanation: Imagine a factory assembly line that produces widgets at a steady pace. Maintaining a constant rate of production ensures that the factory operates efficiently and meets demand.
3. Environmental Science: Understanding zero order kinetics can help in modeling the degradation of pollutants in the environment. Certain degradation processes may follow zero order kinetics, allowing for better predictions of pollutant concentrations over time.
Illustrative Explanation: Picture a lake where pollutants are being removed at a constant rate. Regardless of how much pollution is present, the cleanup process (degradation) occurs steadily until the pollutants are eliminated.
Challenges and Limitations
While zero order reactions provide valuable insights, there are challenges and limitations to consider:
1. Limited Occurrence: Zero order reactions are less common than first and second order reactions. They typically occur under specific conditions, such as enzyme saturation or constant environmental factors.
Illustrative Explanation: Think of zero order reactions as rare gems in a treasure chest. While they are valuable, they are not as frequently encountered as more common types of reactions.
2. Complexity in Real Systems: In real-world systems, reactions may not strictly adhere to zero order kinetics due to varying concentrations, environmental factors, or competing reactions. This complexity can complicate predictions and analyses.
Illustrative Explanation: Imagine a busy intersection where multiple roads converge. The flow of traffic (reaction) may be influenced by various factors, making it challenging to predict the exact behavior of vehicles (reactants).
3. Measurement Challenges: Accurately measuring the rate of zero order reactions can be difficult, especially when dealing with small concentrations or rapid reactions. Precise experimental conditions are necessary to obtain reliable data.
Illustrative Explanation: Think of measuring the flow of a small stream (reaction rate) with a delicate instrument. Any disturbance (experimental error) can affect the readings, making it challenging to obtain accurate measurements.
Conclusion
In conclusion, zero order reactions represent a unique category of chemical reactions characterized by a constant rate that is independent of reactant concentration. Understanding the principles of zero order kinetics is essential for various applications in pharmaceuticals, chemical manufacturing, and environmental science. By recognizing the characteristics, mathematical representations, and real-world implications of zero order reactions, scientists and researchers can better predict and control chemical processes. As we continue to explore the complexities of chemical kinetics, the study of zero order reactions will remain a vital area of research, contributing to advancements in science and technology. Through ongoing investigation and experimentation, we can deepen our understanding of these fascinating reactions and their role in the broader context of chemical behavior.