Avogadro’s Hypothesis is a fundamental principle in chemistry that plays a crucial role in understanding the behavior of gases and the relationships between the quantities of substances involved in chemical reactions. Formulated by the Italian scientist Amedeo Avogadro in the early 19th century, this hypothesis provides a clear and concise explanation of the relationship between the volume of a gas and the number of particles it contains. This article will explore Avogadro’s Hypothesis in detail, including its definition, implications, mathematical formulation, and applications, along with illustrative explanations to enhance understanding.
What is Avogadro’s Hypothesis?
Definition
Avogadro’s Hypothesis states that equal volumes of gases, at the same temperature and pressure, contain an equal number of molecules or particles, regardless of the type of gas. This principle implies that the volume of a gas is directly proportional to the number of moles of gas present, provided that the temperature and pressure remain constant.
- Illustrative Explanation: Imagine two balloons filled with different gases, such as oxygen and nitrogen, both at the same temperature and pressure. According to Avogadro’s Hypothesis, if the balloons have the same volume, they contain the same number of gas molecules, even though the gases are different. This concept is akin to saying that two containers of the same size can hold the same number of marbles, regardless of the color or type of marble.
Historical Context
Amedeo Avogadro proposed his hypothesis in 1811, during a time when the understanding of gases and their behavior was still developing. His work was built upon the earlier contributions of scientists such as Robert Boyle, Jacques Charles, and Joseph Louis Gay-Lussac, who studied the relationships between pressure, volume, and temperature of gases. Avogadro’s Hypothesis provided a unifying framework for these observations, leading to the concept of the mole and the development of modern stoichiometry.
Implications of Avogadro’s Hypothesis
Avogadro’s Hypothesis has several important implications in the field of chemistry, particularly in the study of gases and chemical reactions. Some of the key implications include:
1. Molar Volume of Gases
At standard temperature and pressure (STP), which is defined as 0 degrees Celsius (273.15 K) and 1 atmosphere (101.3 kPa) of pressure, one mole of any ideal gas occupies a volume of approximately 22.4 liters. This volume is known as the molar volume of a gas.
- Illustrative Explanation: Picture a large container filled with helium gas. If you have one mole of helium, it will occupy a volume of about 22.4 liters at STP. This means that regardless of the type of gas, one mole will always occupy this same volume under these conditions, similar to how a specific size of a box can hold a certain number of items, regardless of what those items are.
2. Stoichiometry of Gaseous Reactions
Avogadro’s Hypothesis allows chemists to relate the volumes of gases involved in chemical reactions. For example, if a reaction produces gas, the volume of the gas produced can be directly related to the volume of the reactants, provided that the temperature and pressure are constant.
- Illustrative Explanation: Consider the reaction between hydrogen and oxygen to produce water vapor:
![]()
According to Avogadro’s Hypothesis, if you start with 2 liters of hydrogen gas and 1 liter of oxygen gas, you will produce 2 liters of water vapor, assuming all gases are at the same temperature and pressure. This is similar to a recipe where specific amounts of ingredients yield a predictable amount of product.
3. Determining Molecular Formulas
Avogadro’s Hypothesis is instrumental in determining the molecular formulas of gases. By measuring the volume of a gas and knowing the conditions under which it was measured, chemists can calculate the number of moles and, consequently, the number of molecules present.
- Illustrative Explanation: Imagine you have a gas sample that occupies a volume of 44.8 liters at STP. Using Avogadro’s Hypothesis, you can determine that this volume corresponds to 2 moles of gas (since 44.8 L / 22.4 L/mol = 2 mol). If you know the gas is carbon dioxide (CO₂), you can conclude that you have 2 moles of CO₂ molecules.
Mathematical Formulation of Avogadro’s Hypothesis
Avogadro’s Hypothesis can be expressed mathematically in terms of the relationship between volume and the number of moles of gas. The relationship can be summarized as follows:
![]()
Where:
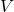 = volume of the gas
= volume of the gas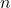 = number of moles of the gas
= number of moles of the gas
This relationship can be expressed in a more formal equation:
![]()
Where:
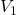 and
and 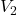 are the volumes of two different gases
are the volumes of two different gases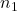 and
and 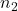 are the corresponding number of moles of those gases
are the corresponding number of moles of those gases
This equation indicates that the ratio of the volumes of two gases is equal to the ratio of the number of moles of those gases when temperature and pressure are constant.
Example Calculation
Suppose you have 3 moles of nitrogen gas (![]() ) at STP. To find the volume occupied by this gas, you can use the molar volume:
) at STP. To find the volume occupied by this gas, you can use the molar volume:
![]()
Substituting the values:
![]()
This calculation shows that 3 moles of nitrogen gas occupy a volume of 67.2 liters at STP.
Applications of Avogadro’s Hypothesis
Avogadro’s Hypothesis has numerous applications in chemistry and related fields. Some notable applications include:
1. Gas Stoichiometry in Chemical Reactions
Avogadro’s Hypothesis is essential for calculating the volumes of gases involved in chemical reactions. It allows chemists to predict the amounts of reactants and products based on their gaseous states.
- Illustrative Explanation: In a laboratory, a chemist might conduct a reaction involving gases. By applying Avogadro’s Hypothesis, they can determine how much of each gas is needed to achieve a desired reaction outcome, similar to how a chef measures ingredients for a recipe.
2. Determining Molecular Mass
By measuring the volume of a gas and knowing its mass, chemists can calculate the molar mass of the gas. This is particularly useful for gases that are difficult to isolate in solid or liquid form.
- Illustrative Explanation: Imagine a chemist weighing a gas sample that occupies 11.2 liters at STP and has a mass of 16 grams. Using Avogadro’s Hypothesis, they can determine the molar mass of the gas by calculating how many moles are present and then finding the mass per mole.
3. Understanding Ideal Gas Behavior
Avogadro’s Hypothesis is a cornerstone of the ideal gas law, which relates pressure, volume, temperature, and the number of moles of a gas. The ideal gas law is expressed as:
![]()
Where:
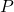 = pressure of the gas
= pressure of the gas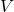 = volume of the gas
= volume of the gas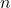 = number of moles of the gas
= number of moles of the gas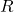 = ideal gas constant
= ideal gas constant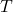 = temperature in Kelvin
= temperature in Kelvin
This law incorporates Avogadro’s Hypothesis by showing how the volume of a gas is directly proportional to the number of moles.
- Illustrative Explanation: Consider a balloon filled with air. If you increase the temperature of the air inside the balloon while keeping the pressure constant, the volume of the balloon will expand. This behavior can be predicted using the ideal gas law, which is grounded in Avogadro’s Hypothesis.
4. Applications in Industry
Avogadro’s Hypothesis is applied in various industrial processes, including the production of gases, chemical manufacturing, and environmental monitoring. Understanding gas volumes and their relationships is crucial for optimizing production and ensuring safety.
- Illustrative Explanation: In a chemical plant, engineers may need to monitor the volumes of gases produced during a reaction. By applying Avogadro’s Hypothesis, they can ensure that the correct amounts of reactants are used, similar to how a factory manager oversees the assembly line to maintain efficiency.
Conclusion
Avogadro’s Hypothesis is a fundamental principle in chemistry that provides valuable insights into the behavior of gases and their relationships in chemical reactions. By establishing that equal volumes of gases contain an equal number of molecules at the same temperature and pressure, Avogadro’s Hypothesis has paved the way for advancements in stoichiometry, gas laws, and molecular theory. Its applications span a wide range of fields, from laboratory research to industrial processes, making it an essential concept for anyone studying or working in the sciences. As we continue to explore the intricacies of gas behavior and chemical reactions, Avogadro’s Hypothesis will remain a cornerstone of our understanding of the molecular world.