Boltzmann’s constant is a fundamental physical constant that plays a crucial role in statistical mechanics, thermodynamics, and the understanding of the microscopic behavior of particles in a system. Named after the Austrian physicist Ludwig Boltzmann, this constant bridges the macroscopic and microscopic worlds, linking temperature to energy at the atomic and molecular levels. This article aims to provide an exhaustive overview of Boltzmann’s constant, detailing its definition, significance, derivation, applications, and illustrative explanations of each concept.
Understanding Boltzmann’s Constant
1. Definition of Boltzmann’s Constant
Boltzmann’s constant, denoted by the symbol ![]() (or sometimes
(or sometimes ![]() ), is defined as the proportionality factor that relates the average kinetic energy of particles in a gas to the temperature of the gas. The value of Boltzmann’s constant is approximately:
), is defined as the proportionality factor that relates the average kinetic energy of particles in a gas to the temperature of the gas. The value of Boltzmann’s constant is approximately:
![]()
Where:
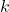 is Boltzmann’s constant,
is Boltzmann’s constant,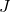 is joules (the unit of energy),
is joules (the unit of energy),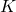 is kelvins (the unit of temperature).
is kelvins (the unit of temperature).- Illustrative Explanation: Imagine a group of children playing a game of tag. The average speed at which they run can be thought of as their kinetic energy. If the temperature of the environment increases (like a sunny day), the children might run faster. Boltzmann’s constant helps us quantify this relationship between the average speed (kinetic energy) of the children (particles) and the temperature of the day.
2. Significance of Boltzmann’s Constant
Boltzmann’s constant is significant for several reasons:
a. Linking Macroscopic and Microscopic Worlds
Boltzmann’s constant serves as a bridge between the macroscopic properties of materials (like temperature and pressure) and the microscopic behavior of individual particles. It allows physicists to understand how the collective behavior of many particles leads to observable phenomena.
- Illustrative Explanation: Think of a large crowd at a concert. While each individual may behave differently, the overall energy and excitement of the crowd can be described in terms of temperature and sound levels. Boltzmann’s constant helps us relate the individual behaviors of particles to the overall properties of the system.
b. Statistical Mechanics
In statistical mechanics, Boltzmann’s constant is used to derive the Boltzmann distribution, which describes the distribution of particles over various energy states in a system at thermal equilibrium. This distribution is fundamental for understanding thermodynamic properties.
- Illustrative Explanation: Imagine a jar filled with different colored marbles representing particles at various energy levels. Boltzmann’s constant helps us understand how many marbles (particles) will be found at each energy level when the jar is shaken (thermal equilibrium).
c. Thermodynamics
Boltzmann’s constant plays a crucial role in thermodynamics, particularly in the formulation of the second law of thermodynamics and the concept of entropy. It helps quantify the amount of disorder or randomness in a system.
- Illustrative Explanation: Picture a messy room with toys scattered everywhere. The more disordered the room, the higher the entropy. Boltzmann’s constant helps us measure this disorder at the microscopic level, relating it to the temperature of the system.
3. Derivation of Boltzmann’s Constant
The derivation of Boltzmann’s constant involves several key concepts from statistical mechanics and thermodynamics:
a. Kinetic Theory of Gases
The kinetic theory of gases describes how gas particles behave in terms of their motion and collisions. According to this theory, the average kinetic energy of gas particles is directly proportional to the temperature of the gas.
- Illustrative Explanation: Imagine a balloon filled with air. As you heat the balloon, the air molecules inside move faster and collide more frequently with the walls of the balloon. The average speed of these molecules is related to the temperature of the gas, illustrating the connection between kinetic energy and temperature.
b. Average Kinetic Energy
The average kinetic energy (![]() ) of a single particle in a gas can be expressed as:
) of a single particle in a gas can be expressed as:
![]()
Where:
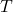 is the absolute temperature in kelvins.
is the absolute temperature in kelvins.
This equation shows that the average kinetic energy of a particle is proportional to the temperature, with Boltzmann’s constant as the proportionality factor.
- Illustrative Explanation: Think of a group of runners in a race. The average speed of the runners increases as the temperature rises on a hot day. Boltzmann’s constant quantifies this relationship, linking the average speed (kinetic energy) to the temperature of the environment.
c. Entropy and Statistical Mechanics
Boltzmann’s constant is also central to the definition of entropy (![]() ), which quantifies the amount of disorder in a system. The relationship is given by:
), which quantifies the amount of disorder in a system. The relationship is given by:
![]()
Where:
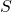 is the entropy,
is the entropy,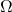 is the number of microstates corresponding to a given macrostate.
is the number of microstates corresponding to a given macrostate.
This equation shows that entropy increases with the number of ways particles can be arranged in a system.
- Illustrative Explanation: Imagine a box filled with different colored balls. The more ways you can arrange the balls (microstates), the messier the box becomes (higher entropy). Boltzmann’s constant helps us quantify this relationship between arrangement and disorder.
4. Applications of Boltzmann’s Constant
Boltzmann’s constant has numerous applications across various fields, including:
a. Statistical Mechanics
In statistical mechanics, Boltzmann’s constant is used to derive important equations and distributions, such as the Maxwell-Boltzmann distribution, which describes the distribution of speeds of particles in a gas.
- Illustrative Explanation: Picture a race with runners of different speeds. The Maxwell-Boltzmann distribution helps us understand how many runners (particles) are running at each speed (energy level) at a given temperature.
b. Thermodynamics
In thermodynamics, Boltzmann’s constant is used to calculate changes in entropy and to understand the behavior of systems in thermal equilibrium. It is essential for analyzing heat engines and refrigerators.
- Illustrative Explanation: Think of a refrigerator. Boltzmann’s constant helps us understand how energy is transferred and how the refrigerator maintains a cool environment by removing heat from inside.
c. Cosmology and Astrophysics
In cosmology, Boltzmann’s constant is used to understand the behavior of particles in the early universe and the formation of structures like galaxies. It helps explain phenomena such as cosmic microwave background radiation.
- Illustrative Explanation: Imagine a hot soup cooling down. As the soup cools, particles begin to settle and form clumps. Similarly, Boltzmann’s constant helps us understand how particles in the early universe cooled and formed galaxies over time.
d. Material Science
In material science, Boltzmann’s constant is used to study the thermal properties of materials, including heat capacity and thermal conductivity. It helps researchers understand how materials respond to changes in temperature.
- Illustrative Explanation: Consider a metal rod heated at one end. Boltzmann’s constant helps us analyze how quickly heat travels through the rod and how the material expands as it heats up.
Conclusion
In conclusion, Boltzmann’s constant is a fundamental physical constant that plays a crucial role in linking the macroscopic properties of materials to their microscopic behavior. By exploring its definition, significance, derivation, applications, and illustrative explanations, we can appreciate the importance of Boltzmann’s constant in various fields, including statistical mechanics, thermodynamics, cosmology, and material science. Understanding this constant not only enhances our knowledge of the behavior of particles and energy but also provides valuable insights into the fundamental principles that govern the universe. As research in physics and related fields continues to evolve, Boltzmann’s constant will remain a critical tool for analyzing and understanding the complex interactions between temperature, energy, and the behavior of matter at the atomic and molecular levels.