Capillarity, also known as capillary action, is a fascinating phenomenon that describes the ability of a liquid to flow in narrow spaces without the assistance of external forces, such as gravity. This behavior is particularly evident in small tubes or porous materials and is a crucial concept in various scientific fields, including physics, chemistry, biology, and engineering. This article will provide an in-depth examination of capillarity, covering its definition, underlying principles, factors affecting capillarity, applications, and illustrative explanations to enhance understanding.
1. Definition of Capillarity
Capillarity refers to the ability of a liquid to rise or fall in a narrow space, such as a thin tube or a porous material, due to the interplay of cohesive and adhesive forces. When a liquid is in contact with a solid surface, the interactions between the liquid molecules and the solid surface can lead to a rise or depression of the liquid level in the narrow space. This phenomenon is commonly observed in everyday life, such as when a paper towel absorbs water or when water rises in a thin straw.
Illustrative Explanation: Imagine a thin straw placed in a glass of water. When you observe the water level inside the straw, you will notice that it rises higher than the water level in the glass. This is capillarity in action, where the adhesive forces between the water molecules and the straw’s surface cause the water to climb up the straw.
2. Underlying Principles of Capillarity
Capillarity is primarily governed by two types of intermolecular forces: cohesive forces and adhesive forces.
- Cohesive Forces: These are the attractive forces between molecules of the same substance. In the case of water, cohesive forces arise from hydrogen bonding between water molecules. These forces tend to hold the liquid together and resist separation.
- Adhesive Forces: These are the attractive forces between molecules of different substances. For example, when water comes into contact with a glass surface, adhesive forces between the water molecules and the glass molecules cause the water to cling to the glass.
The balance between cohesive and adhesive forces determines the behavior of the liquid in narrow spaces. If the adhesive forces are stronger than the cohesive forces, the liquid will rise in the narrow space. Conversely, if cohesive forces dominate, the liquid will be depressed.
Illustrative Explanation: Consider a glass of water and a piece of wax paper. When you dip the wax paper into the water, the water does not climb up the paper because the cohesive forces between the water molecules are stronger than the adhesive forces between the water and the wax. In contrast, if you dip a clean glass rod into the water, the water will rise along the rod due to the stronger adhesive forces between the water and the glass.
3. Factors Affecting Capillarity
Several factors influence the extent of capillary action, including:
- Diameter of the Capillary Tube: The diameter of the tube plays a significant role in capillarity. In narrower tubes, the surface area in contact with the liquid is larger relative to the volume of the liquid, leading to a more pronounced capillary rise. Conversely, in wider tubes, the effect is diminished.
- Surface Tension: Surface tension is the property of a liquid that causes its surface to behave like a stretched elastic membrane. Liquids with higher surface tension, such as water, exhibit stronger capillary action compared to liquids with lower surface tension, such as oil.
- Contact Angle: The contact angle is the angle formed between the liquid surface and the solid surface at the point of contact. A smaller contact angle indicates stronger adhesive forces, leading to greater capillary rise. Conversely, a larger contact angle suggests weaker adhesive forces and reduced capillarity.
- Nature of the Liquid and Solid: The chemical properties of the liquid and the solid surface also affect capillarity. For example, polar liquids (like water) tend to exhibit stronger adhesive forces with polar surfaces (like glass) compared to non-polar liquids (like oil) with non-polar surfaces (like wax).
Illustrative Explanation: Picture two different tubes: one is a thin straw, and the other is a wide glass. When you place both in a glass of water, the water will rise significantly higher in the thin straw than in the wide glass. This is because the narrow diameter of the straw enhances the adhesive forces between the water and the straw, leading to a greater capillary rise.
4. Applications of Capillarity
Capillarity has numerous practical applications across various fields:
- Soil Moisture Movement: In agriculture, capillarity plays a crucial role in the movement of water through soil. Water moves through the tiny pores in the soil due to capillary action, allowing plants to absorb moisture and nutrients.
- Ink in Pens: Fountain pens and ink pens utilize capillarity to draw ink from the reservoir to the nib. The narrow channels in the nib create a capillary effect that allows the ink to flow smoothly onto the paper.
- Absorbent Materials: Capillarity is the principle behind the functioning of absorbent materials, such as paper towels and sponges. These materials have tiny pores that allow liquids to be drawn in through capillary action.
- Medical Applications: Capillary action is utilized in various medical devices, such as capillary blood collection tubes and microfluidic devices, which rely on the movement of small volumes of liquid through narrow channels.
Illustrative Explanation: Think of a sponge soaking up water. When you place a dry sponge in a puddle, the water quickly rises into the sponge due to capillary action. The tiny pores in the sponge act like capillary tubes, drawing the water in and allowing the sponge to absorb it.
5. Capillary Rise and Fall
Capillary action can lead to both rise and fall of liquids in different contexts. The rise occurs in situations where adhesive forces dominate, while fall occurs when cohesive forces are stronger.
- Capillary Rise: This occurs when a liquid, such as water, rises in a narrow tube or porous material. The height to which the liquid rises can be calculated using the formula:
![]()
Where:
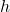 is the height of the liquid column.
is the height of the liquid column.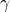 is the surface tension of the liquid.
is the surface tension of the liquid.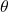 is the contact angle.
is the contact angle.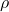 is the density of the liquid.
is the density of the liquid.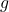 is the acceleration due to gravity.
is the acceleration due to gravity. is the radius of the capillary tube.
is the radius of the capillary tube.- Capillary Fall: This occurs when a liquid is drawn down into a narrow space, such as when mercury is placed in a glass tube. In this case, the cohesive forces between mercury molecules are stronger than the adhesive forces between mercury and glass, causing the mercury to be depressed in the tube.
Illustrative Explanation: Imagine a glass tube filled with water. If you place a thin straw in the water, the water will rise inside the straw due to capillary action. Now, if you replace the water with mercury, you will notice that the mercury level drops inside the straw because the cohesive forces between mercury molecules are stronger than the adhesive forces with the glass.
Conclusion
Capillarity is a remarkable phenomenon that illustrates the interplay of cohesive and adhesive forces in liquids. By exploring the definitions, underlying principles, factors affecting capillarity, applications, and the concepts of capillary rise and fall, we gain a deeper appreciation for its significance in both natural and technological contexts. From the movement of water in soil to the functioning of everyday objects like pens and sponges, capillarity plays a vital role in our daily lives. As we continue to study and apply these principles, we unlock new possibilities for innovation and understanding in the world of fluids and materials.