The Carnot engine is a theoretical thermodynamic cycle that serves as a benchmark for the efficiency of all heat engines. Named after the French physicist Sadi Carnot, who first described it in 1824, the Carnot engine provides critical insights into the principles of thermodynamics and the limits of energy conversion. Understanding the Carnot engine is essential for anyone interested in physics, engineering, or environmental science, as it lays the groundwork for modern thermodynamic theory and the development of more efficient energy systems. This article will explore the Carnot engine’s principles, operation, efficiency, and significance, providing detailed explanations and illustrative examples to enhance understanding.
What is a Carnot Engine?
Definition
A Carnot engine is an idealized heat engine that operates on the Carnot cycle, which consists of four reversible processes: two isothermal (constant temperature) processes and two adiabatic (no heat transfer) processes. The Carnot engine is designed to convert heat energy from a high-temperature reservoir into work while rejecting some heat to a low-temperature reservoir. It represents the maximum possible efficiency that any heat engine can achieve when operating between two temperature limits.
- Illustrative Explanation: Imagine a perfectly efficient machine that can convert heat from a hot source, like a boiling pot of water, into mechanical work, such as turning a wheel. The Carnot engine is a theoretical model of such a machine, demonstrating the best possible performance under ideal conditions.
Components of a Carnot Engine
1. Heat Reservoirs: The Carnot engine operates between two heat reservoirs: a hot reservoir (source) at a high temperature (![]() ) and a cold reservoir (sink) at a lower temperature (
) and a cold reservoir (sink) at a lower temperature (![]() ). The heat engine absorbs heat (
). The heat engine absorbs heat (![]() ) from the hot reservoir and rejects heat (
) from the hot reservoir and rejects heat (![]() ) to the cold reservoir.
) to the cold reservoir.
2. Working Substance: The working substance is the fluid (often a gas) that undergoes the thermodynamic processes in the engine. It absorbs heat, expands, does work, and then releases heat.
3. Mechanical Work Output: The work output (![]() ) of the Carnot engine is the difference between the heat absorbed from the hot reservoir and the heat rejected to the cold reservoir:
) of the Carnot engine is the difference between the heat absorbed from the hot reservoir and the heat rejected to the cold reservoir:
![]()
- Illustrative Explanation: Think of the hot reservoir as a battery that provides energy to the engine. The engine uses some of this energy to perform work, while the rest is released to the cold reservoir, similar to how a battery discharges energy to power a device.
The Carnot Cycle
The Carnot cycle consists of four distinct processes that occur in a closed system, allowing the working substance to undergo a series of transformations. These processes are:
1. Isothermal Expansion (Process 1-2)
In this process, the working substance (gas) is placed in contact with the hot reservoir at temperature ![]() . The gas absorbs heat (
. The gas absorbs heat (![]() ) from the reservoir while expanding isothermally (at constant temperature). As the gas expands, it does work on the surroundings.
) from the reservoir while expanding isothermally (at constant temperature). As the gas expands, it does work on the surroundings.
- Illustrative Explanation: Imagine a balloon filled with air. If you place the balloon in a warm environment, the air inside the balloon absorbs heat, causing it to expand. The balloon pushes against the surrounding air, doing work as it expands.
2. Adiabatic Expansion (Process 2-3)
During this process, the gas continues to expand, but it is now insulated from the heat reservoir, meaning no heat is exchanged with the surroundings. As the gas expands adiabatically, it does work on the surroundings, causing its temperature to decrease.
- Illustrative Explanation: Picture a gas-filled piston that is allowed to expand without any heat exchange with the environment. As the gas expands, it cools down because it is doing work on the piston without receiving any heat, similar to how a bicycle pump feels cooler when you compress it.
3. Isothermal Compression (Process 3-4)
In this process, the working substance is placed in contact with the cold reservoir at temperature ![]() . The gas is compressed isothermally, meaning it releases heat (
. The gas is compressed isothermally, meaning it releases heat (![]() ) to the cold reservoir while maintaining a constant temperature. The work is done on the gas during this compression.
) to the cold reservoir while maintaining a constant temperature. The work is done on the gas during this compression.
- Illustrative Explanation: Imagine squeezing the same balloon in a cold environment. As you compress the balloon, the air inside releases heat to the surroundings, and the temperature remains constant while the volume decreases.
4. Adiabatic Compression (Process 4-1)
During this final process, the gas is again insulated from the heat reservoir. The gas is compressed adiabatically, which increases its temperature without any heat exchange. The work done on the gas raises its internal energy, preparing it for the next cycle.
- Illustrative Explanation: Think of the gas in the piston being compressed further without any heat exchange. As you push down on the piston, the gas temperature rises due to the work being done on it, similar to how a bicycle pump heats up when you compress it rapidly.
Efficiency of the Carnot Engine
The efficiency (![]() ) of a Carnot engine is defined as the ratio of the work output to the heat input from the hot reservoir. It can be expressed mathematically as:
) of a Carnot engine is defined as the ratio of the work output to the heat input from the hot reservoir. It can be expressed mathematically as:
![]()
Since the heat exchanged is proportional to the temperatures of the reservoirs, the efficiency can also be expressed in terms of the absolute temperatures:
![]()
Where:
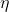 = efficiency (dimensionless, expressed as a fraction)
= efficiency (dimensionless, expressed as a fraction)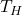 = absolute temperature of the hot reservoir (in Kelvin)
= absolute temperature of the hot reservoir (in Kelvin)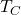 = absolute temperature of the cold reservoir (in Kelvin)
= absolute temperature of the cold reservoir (in Kelvin)
Key Points about Efficiency
1. Maximum Efficiency: The Carnot engine represents the maximum efficiency that any heat engine can achieve when operating between two temperature limits. No real engine can exceed this efficiency due to irreversibilities and losses in practical systems.
2. Dependence on Temperature: The efficiency of the Carnot engine increases as the temperature difference between the hot and cold reservoirs increases. A larger temperature difference allows for more work to be extracted from the heat input.
- Illustrative Explanation: Imagine two engines: one operating between a boiling pot of water (100°C) and ice water (0°C) and another operating between molten lava (1200°C) and ice water. The engine with the larger temperature difference (the lava engine) will be more efficient, similar to how a steep hill allows a roller coaster to gain more speed.
Significance of the Carnot Engine
The Carnot engine is significant for several reasons:
1. Foundation of Thermodynamics
The Carnot engine laid the groundwork for the second law of thermodynamics, which states that no heat engine can be 100% efficient. It established the concept of entropy and the idea that energy transformations are inherently inefficient due to the tendency of systems to move toward equilibrium.
- Illustrative Explanation: Think of a perfectly efficient engine as a perpetual motion machine. The Carnot engine demonstrates that such machines are impossible, as energy will always be lost to the surroundings, similar to how a leaky faucet cannot fill a bucket without losing water.
2. Benchmark for Real Engines
The Carnot engine serves as a benchmark against which the performance of real heat engines can be measured. Engineers and scientists use the Carnot efficiency as a goal for improving the design and operation of practical engines.
- Illustrative Explanation: Imagine a car engine that is designed to be as efficient as possible. Engineers will compare its performance to the Carnot engine to identify areas for improvement, much like how athletes strive to break world records.
3. Understanding Heat Transfer
The principles of the Carnot engine help in understanding heat transfer processes and the limitations of energy conversion in various systems, including power plants, refrigeration, and air conditioning.
- Illustrative Explanation: Consider a power plant that generates electricity from heat. By applying the principles of the Carnot engine, engineers can optimize the heat transfer processes to maximize efficiency, similar to how a chef adjusts cooking times and temperatures for the best results.
Applications of the Carnot Engine Concept
While the Carnot engine itself is a theoretical construct, its principles have practical applications in various fields:
1. Heat Engines
The design and analysis of heat engines, such as steam engines, internal combustion engines, and gas turbines, are influenced by the Carnot cycle. Engineers strive to approach Carnot efficiency in their designs.
- Illustrative Explanation: Imagine a modern car engine. Engineers use the Carnot cycle as a guideline to improve fuel efficiency and reduce emissions, similar to how a chef follows a recipe to create a delicious dish.
2. Refrigeration and Heat Pumps
The principles of the Carnot cycle are also applied in refrigeration and heat pump systems, where heat is transferred from a cold reservoir to a hot reservoir. Understanding the Carnot cycle helps optimize these systems for better performance.
- Illustrative Explanation: Think of a refrigerator. Engineers use the Carnot principles to design systems that efficiently remove heat from the inside of the fridge, keeping your food cold while minimizing energy consumption.
3. Renewable Energy Systems
The Carnot engine concept is relevant in the development of renewable energy systems, such as solar thermal power plants, where heat is converted into electricity. Understanding the efficiency limits helps in designing more effective systems.
- Illustrative Explanation: Imagine a solar power plant that uses mirrors to concentrate sunlight and generate steam. Engineers apply Carnot principles to maximize the conversion of solar energy into electricity, similar to how a gardener optimizes sunlight exposure for plant growth.
Conclusion
The Carnot engine is a fundamental concept in thermodynamics that illustrates the principles of heat transfer, energy conversion, and efficiency. By understanding the Carnot cycle, its components, and its significance, we gain valuable insights into the limitations and potential of heat engines. The Carnot engine serves as a benchmark for real-world applications, guiding engineers and scientists in their quest for more efficient energy systems. As we continue to explore the intricacies of thermodynamics and energy conversion, the principles of the Carnot engine will remain integral to our understanding of the physical world and the development of sustainable technologies.