The electron is one of the fundamental particles that make up atoms, playing a crucial role in the structure of matter and the behavior of electromagnetic forces. Understanding the charge of the electron is essential for grasping concepts in physics, chemistry, and various fields of engineering. This article will provide a comprehensive overview of the charge of the electron, including its definition, properties, historical context, significance, and applications, along with illustrative explanations to enhance understanding.
1. Overview of the Electron
1.1 Definition of the Electron
An electron is a subatomic particle with a negative electric charge, denoted as ![]() elementary charge (e). It is one of the primary constituents of atoms, alongside protons and neutrons. Electrons are classified as leptons, a family of fundamental particles that do not experience the strong nuclear force.
elementary charge (e). It is one of the primary constituents of atoms, alongside protons and neutrons. Electrons are classified as leptons, a family of fundamental particles that do not experience the strong nuclear force.
Illustration: Think of an electron as a tiny, negatively charged ball that orbits around the nucleus of an atom, similar to how planets orbit around the sun. Just as planets have gravitational forces acting on them, electrons are influenced by electromagnetic forces due to their charge.
1.2 Properties of the Electron
- Charge: The charge of an electron is approximately
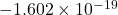 coulombs. This negative charge is fundamental to the behavior of atoms and molecules.
coulombs. This negative charge is fundamental to the behavior of atoms and molecules. - Mass: The mass of an electron is about
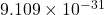 kilograms, which is approximately 1/1836 the mass of a proton. This small mass allows electrons to move freely and participate in chemical reactions.
kilograms, which is approximately 1/1836 the mass of a proton. This small mass allows electrons to move freely and participate in chemical reactions. - Spin: Electrons possess a property called spin, which is a form of intrinsic angular momentum. The spin of an electron is
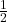 , classifying it as a fermion.
, classifying it as a fermion.
Illustration: Visualize the electron’s charge as a magnet with a negative pole. Just as magnets have poles that attract or repel each other, the negative charge of an electron interacts with the positive charge of protons, leading to the formation of atoms.
2. Historical Context
2.1 Discovery of the Electron
The electron was discovered in 1897 by British physicist J.J. Thomson through experiments with cathode rays. Thomson observed that cathode rays were deflected by electric and magnetic fields, indicating that they were composed of negatively charged particles. He concluded that these particles were much smaller than atoms and named them “corpuscles,” later known as electrons.
Illustration: Imagine Thomson as a detective uncovering a hidden treasure. Just as a detective uses clues to find something valuable, Thomson used experiments to reveal the existence of the electron, a fundamental building block of matter.
2.2 Measurement of Charge
The charge of the electron was first measured by American physicist Robert Millikan in his famous oil drop experiment conducted in 1909. Millikan suspended tiny oil droplets in an electric field and measured the force required to balance the gravitational force acting on the droplets. By analyzing the behavior of the droplets, he was able to determine the charge of the electron with remarkable precision.
Illustration: Think of Millikan’s experiment as a balancing act on a seesaw. Just as a seesaw requires equal weights on both sides to remain balanced, Millikan balanced the forces acting on the oil droplets to measure the charge of the electron.
3. Significance of the Charge of the Electron
3.1 Fundamental Role in Atoms
The charge of the electron is crucial for the formation of atoms. Electrons are attracted to the positively charged protons in the nucleus, creating a stable structure. The balance between the number of protons and electrons determines the overall charge of an atom, leading to the formation of neutral atoms or ions.
Illustration: Visualize an atom as a solar system, with the nucleus as the sun and electrons as planets orbiting around it. Just as the gravitational pull of the sun keeps the planets in orbit, the electrostatic attraction between protons and electrons holds the atom together.
3.2 Chemical Bonding
The charge of electrons plays a vital role in chemical bonding. Atoms can gain, lose, or share electrons to achieve a stable electron configuration, leading to the formation of ionic and covalent bonds. The behavior of electrons in chemical reactions is governed by their charge and the principles of electrostatics.
Illustration: Think of chemical bonding as a dance between partners. Just as dancers must connect and synchronize their movements, atoms interact through the transfer or sharing of electrons to form stable compounds.
3.3 Electricity and Conductivity
The movement of electrons is fundamental to the flow of electric current. In conductive materials, such as metals, electrons can move freely, allowing for the conduction of electricity. The charge of the electron is responsible for the behavior of electric circuits and the functioning of electronic devices.
Illustration: Visualize electricity as water flowing through pipes. Just as water moves through pipes to power a water wheel, the flow of electrons through conductive materials generates electric current, powering our homes and devices.
4. Applications of the Charge of the Electron
4.1 Electronics
The charge of the electron is the basis for modern electronics. Transistors, diodes, and integrated circuits rely on the movement and manipulation of electrons to perform various functions, such as amplification, switching, and signal processing.
Illustration: Think of electronic devices as intricate machines powered by tiny workers. Just as workers perform specific tasks to keep a factory running, electrons move through circuits to enable the operation of electronic devices.
4.2 Chemistry and Material Science
Understanding the charge of the electron is essential in chemistry and material science. It helps explain the behavior of atoms and molecules, guiding the design of new materials, catalysts, and chemical reactions.
Illustration: Visualize chemists as chefs experimenting with recipes. Just as chefs adjust ingredients and techniques to create new dishes, chemists manipulate the charge and behavior of electrons to develop innovative materials and reactions.
4.3 Medical Applications
The charge of the electron is also significant in medical applications, such as in imaging techniques like X-rays and MRI. These technologies rely on the interaction of electrons with matter to produce images of the human body.
Illustration: Think of medical imaging as a camera capturing moments in time. Just as a camera uses light to create images, medical imaging techniques utilize the behavior of electrons to visualize internal structures and diagnose conditions.
Conclusion
The charge of the electron is a fundamental concept in physics and chemistry, playing a crucial role in the structure of atoms, chemical bonding, and the flow of electricity. Understanding the properties and significance of the electron’s charge provides valuable insights into the behavior of matter and the principles that govern the natural world.
From its discovery by J.J. Thomson to its applications in modern technology and medicine, the electron continues to be a central focus of scientific research and innovation. By fostering awareness of the charge of the electron, we can better appreciate the intricate processes that shape our understanding of the universe and the technologies that enhance our daily lives.