Charles’ Law is one of the fundamental gas laws that describes the relationship between the volume and temperature of a gas at constant pressure. Formulated by the French scientist Jacques Charles in the late 18th century, this law states that the volume of a given mass of gas is directly proportional to its absolute temperature, provided that the pressure remains constant. This relationship is crucial for understanding how gases behave under varying temperature conditions and has significant implications in both theoretical and practical applications in science and engineering. This article will delve into the definition, mathematical formulation, derivation, applications, and limitations of Charles’ Law, providing a thorough understanding of this essential concept, complete with illustrative explanations to enhance comprehension.
Definition of Charles’ Law
Charles’ Law can be succinctly stated as follows:
At constant pressure, the volume of a gas is directly proportional to its absolute temperature (in Kelvin).
Mathematically, this relationship can be expressed as:
![]()
Where:
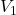 and
and 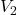 are the initial and final volumes of the gas, respectively.
are the initial and final volumes of the gas, respectively.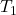 and
and 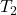 are the initial and final absolute temperatures (in Kelvin) of the gas, respectively.
are the initial and final absolute temperatures (in Kelvin) of the gas, respectively.
Illustrative Explanation: Imagine a balloon filled with air. If you place the balloon in a warm room, the air inside heats up, causing the balloon to expand. This expansion illustrates Charles’ Law: as the temperature increases, so does the volume of the gas inside the balloon, provided the pressure remains constant.
Derivation of Charles’ Law
Charles’ Law can be derived from the Ideal Gas Law, which states:
![]()
Where:
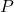 = Pressure
= Pressure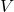 = Volume
= Volume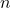 = Number of moles of gas
= Number of moles of gas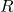 = Ideal gas constant
= Ideal gas constant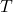 = Absolute temperature in Kelvin
= Absolute temperature in Kelvin
To derive Charles’ Law, we can consider a fixed amount of gas (constant ![]() ) at constant pressure (
) at constant pressure (![]() ). Rearranging the Ideal Gas Law gives:
). Rearranging the Ideal Gas Law gives:
![]()
Since ![]() and
and ![]() are constants, we can express the equation as:
are constants, we can express the equation as:
![]()
This indicates that the volume ![]() is directly proportional to the absolute temperature
is directly proportional to the absolute temperature ![]() when pressure is held constant, which is the essence of Charles’ Law.
when pressure is held constant, which is the essence of Charles’ Law.
Illustrative Explanation: Think of a pot of water on the stove. As you heat the water (increase temperature), the steam (gas) produced expands and takes up more space (volume). The relationship between the heat applied (temperature) and the steam produced (volume) reflects the principles of Charles’ Law.
Applications of Charles’ Law
Charles’ Law has numerous practical applications across various fields, including:
1. Hot Air Balloons
Hot air balloons operate based on the principles of Charles’ Law. When the air inside the balloon is heated, it expands, causing the volume of the gas to increase. Since the pressure remains constant, the balloon rises as the density of the hot air becomes less than that of the cooler air outside.
Illustrative Explanation: Imagine a hot air balloon as a giant balloon filled with warm air. As the air heats up, it expands, making the balloon lighter than the surrounding cooler air, allowing it to float upward.
2. Weather Balloons
Meteorologists use weather balloons to gather data about atmospheric conditions at various altitudes. As the balloon ascends, the temperature decreases, causing the gas inside to contract. Understanding this relationship helps meteorologists predict weather patterns and atmospheric behavior.
Illustrative Explanation: Picture a weather balloon rising into the sky. As it goes higher, the air gets colder, and the gas inside the balloon contracts. This behavior helps scientists understand how temperature changes with altitude.
3. Refrigeration and Air Conditioning
In refrigeration and air conditioning systems, gases are compressed and expanded to absorb and release heat. Charles’ Law helps engineers design systems that efficiently manage temperature and volume changes in refrigerants, ensuring effective cooling.
Illustrative Explanation: Think of a refrigerator as a magic box that keeps your food cold. The refrigerant gas inside expands and contracts as it moves through the system, absorbing heat from the inside and releasing it outside, all while following the principles of Charles’ Law.
4. Breathing Mechanism
The human respiratory system also exemplifies Charles’ Law. When we inhale, the diaphragm contracts, increasing the volume of the thoracic cavity. This decrease in pressure allows air to flow into the lungs, where the temperature of the inhaled air can affect its volume.
Illustrative Explanation: Imagine your lungs as balloons. When you take a deep breath, your diaphragm pulls down, making more space for air (increasing volume). The air you breathe in expands to fill that space, demonstrating Charles’ Law in action.
Limitations of Charles’ Law
While Charles’ Law is a valuable tool for understanding gas behavior, it has limitations:
1. Ideal Gas Assumption
Charles’ Law assumes that gases behave ideally, meaning that gas molecules do not interact with each other and occupy no volume. However, real gases deviate from this behavior under high pressure and low temperature, where intermolecular forces and molecular volume become significant.
Illustrative Explanation: Imagine a group of friends in a small car (real gas). When the car is full (high pressure), they start to feel cramped (deviations from ideal behavior). In contrast, when they are in a spacious van (ideal gas), they can move freely without feeling crowded.
2. Limited Temperature Range
Charles’ Law is most accurate at moderate temperatures and pressures. At extremely low temperatures, gases can condense into liquids, and at high temperatures, gases may dissociate into their constituent atoms, leading to deviations from the law.
Illustrative Explanation: Think of a soda can. When you shake it (increase pressure), the gas inside can become liquid (deviation from gas behavior). Similarly, at very low temperatures, gases can freeze, making Charles’ Law less applicable.
3. Non-ideal Gas Behavior
Certain gases, such as water vapor and carbon dioxide, exhibit non-ideal behavior due to strong intermolecular forces. In such cases, more complex equations, such as the Van der Waals equation, are used to describe gas behavior more accurately.
Illustrative Explanation: Imagine a group of friends who are very close (strong intermolecular forces). They interact more than a group of acquaintances (ideal gas), leading to different dynamics. In this case, a more nuanced approach is needed to understand their interactions.
Conclusion
In conclusion, Charles’ Law is a fundamental principle that describes the relationship between the volume and temperature of a gas at constant pressure. By understanding the definition, derivation, applications, and limitations of Charles’ Law, we gain valuable insights into gas behavior and its implications in various scientific fields. The law serves as a cornerstone for further studies in thermodynamics, physical chemistry, and engineering. As we continue to explore the intricacies of gas behavior, we unlock new possibilities for innovation and discovery, ultimately enriching our understanding of the natural world and its complex chemical processes. Through ongoing research and development, the principles of Charles’ Law will continue to play a vital role in shaping the future of science and technology, contributing to solutions that address global challenges and improve our quality of life.