Chemical kinetics is the branch of physical chemistry that studies the rates of chemical reactions and the factors that influence these rates. Understanding chemical kinetics is essential for predicting how quickly a reaction will occur, which is crucial in various fields, including pharmaceuticals, environmental science, and industrial chemistry. This article aims to provide an exhaustive overview of chemical kinetics, detailing its fundamental concepts, reaction rates, mechanisms, factors affecting reaction rates, and applications, along with illustrative explanations of key concepts.
Understanding Chemical Kinetics
1. Definition of Chemical Kinetics
Chemical kinetics is the study of the speed or rate at which chemical reactions occur and the steps involved in the reaction process. It provides insights into how different conditions affect the speed of reactions and helps in understanding the underlying mechanisms.
- Illustrative Explanation: Imagine a race between two cars (the chemical reactions). Just as the speed of the cars determines how quickly they reach the finish line, the rate of a chemical reaction determines how quickly reactants are converted into products.
Fundamental Concepts in Chemical Kinetics
1. Reaction Rate
The reaction rate is defined as the change in concentration of a reactant or product per unit time. It can be expressed mathematically as:
![]()
![]()
Where ![]() and
and ![]() are the concentrations of the reactant and product, respectively, and
are the concentrations of the reactant and product, respectively, and ![]() is time.
is time.
- Illustrative Explanation: Think of a water tank being filled (the reaction). The rate at which the water level rises (the reaction rate) depends on how quickly water is being added (the change in concentration over time). If the water flows in faster, the tank fills up more quickly, just as a faster reaction rate means products are formed more rapidly.
2. Order of Reaction
The order of a reaction is a classification that indicates how the rate of reaction depends on the concentration of reactants. It can be determined experimentally and is expressed as a sum of the powers of the concentration terms in the rate law.
- Illustrative Explanation: Imagine a recipe where the amount of each ingredient affects the final dish’s flavor. If doubling the amount of spice (a reactant) doubles the flavor intensity, the reaction is first-order with respect to that ingredient. If it quadruples the flavor, it’s second-order. Just as the recipe’s outcome depends on ingredient proportions, the reaction rate depends on the concentrations of reactants.
3. Rate Law
The rate law expresses the relationship between the rate of a reaction and the concentration of its reactants. It is generally written in the form:
![]()
Where ![]() is the rate constant,
is the rate constant, ![]() and
and ![]() are the concentrations of the reactants, and
are the concentrations of the reactants, and ![]() and
and ![]() are the orders of the reaction with respect to each reactant.
are the orders of the reaction with respect to each reactant.
- Illustrative Explanation: Think of a formula for a cake (the rate law). The ingredients (reactants) and their amounts (concentrations) determine how quickly the cake bakes (the reaction rate). The rate constant
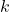 is like the oven temperature; it influences how fast the cake cooks, just as it influences the reaction rate.
is like the oven temperature; it influences how fast the cake cooks, just as it influences the reaction rate.
Factors Affecting Reaction Rates
Several factors can influence the rate of a chemical reaction. Understanding these factors is crucial for controlling and optimizing reactions in various applications.
1. Concentration of Reactants
Increasing the concentration of reactants generally increases the reaction rate. This is because a higher concentration leads to more frequent collisions between reactant molecules.
- Illustrative Explanation: Imagine a crowded room where people are trying to talk (the reactants). If more people enter the room (increased concentration), they are more likely to bump into each other and start conversations (collisions), leading to more discussions (faster reactions).
2. Temperature
Increasing the temperature typically increases the reaction rate. Higher temperatures provide reactant molecules with more kinetic energy, leading to more frequent and energetic collisions.
- Illustrative Explanation: Think of a pot of water on a stove. As the heat increases (temperature), the water molecules move faster and collide more often, leading to quicker boiling (faster reaction). Just as heating speeds up the boiling process, it accelerates chemical reactions.
3. Catalysts
Catalysts are substances that increase the reaction rate without being consumed in the process. They work by providing an alternative reaction pathway with a lower activation energy.
- Illustrative Explanation: Imagine a shortcut on a long road (the catalyst). Taking the shortcut allows you to reach your destination faster without changing the overall journey (the reaction). Just as the shortcut reduces travel time, a catalyst lowers the energy barrier for the reaction, speeding it up.
4. Surface Area
For solid reactants, increasing the surface area can increase the reaction rate. More surface area allows for more collisions between reactant particles.
- Illustrative Explanation: Think of a block of ice (the solid reactant). If you break it into smaller pieces (increased surface area), it melts faster because more ice is exposed to the warmer air (more collisions). Just as breaking the ice speeds up melting, increasing surface area accelerates reactions.
5. Pressure
For reactions involving gases, increasing the pressure can increase the reaction rate. Higher pressure compresses the gas molecules, leading to more frequent collisions.
- Illustrative Explanation: Imagine a balloon filled with air (the gas reactants). If you squeeze the balloon (increase pressure), the air molecules are forced closer together, leading to more collisions and faster reactions. Just as squeezing the balloon increases the pressure, it can enhance the reaction rate in gas-phase reactions.
Reaction Mechanisms
The reaction mechanism is a detailed description of the step-by-step process by which reactants are converted into products. Understanding the mechanism helps chemists design better reactions and predict outcomes.
1. Elementary Steps
Elementary steps are individual reactions that occur in a reaction mechanism. Each step involves a specific arrangement of reactants and products and can be classified as unimolecular, bimolecular, or termolecular based on the number of molecules involved.
- Illustrative Explanation: Think of a relay race (the reaction mechanism). Each runner (elementary step) passes the baton (reactants) to the next runner (products) in a specific order. Just as each runner plays a crucial role in completing the race, each elementary step is essential for the overall reaction.
2. Rate-Determining Step
The rate-determining step is the slowest step in a reaction mechanism, which limits the overall reaction rate. The rate of the entire reaction is determined by this step.
- Illustrative Explanation: Imagine a traffic jam on a highway (the reaction). The slowest car (the rate-determining step) dictates how quickly the entire line of cars can move. Just as the traffic flow is limited by the slowest vehicle, the overall reaction rate is limited by the slowest step in the mechanism.
3. Catalytic Mechanisms
In catalytic reactions, the catalyst participates in the reaction mechanism, often forming intermediate species. Understanding these mechanisms helps in designing more efficient catalysts.
- Illustrative Explanation: Think of a chef (the catalyst) who helps prepare a meal (the reaction). The chef may chop vegetables (form intermediates) and add spices (facilitate steps) to enhance the dish’s flavor. Just as the chef improves the cooking process, catalysts enhance reaction efficiency.
Applications of Chemical Kinetics
Chemical kinetics has numerous applications across various fields, including pharmaceuticals, environmental science, and industrial processes.
1. Pharmaceutical Development
In drug development, understanding the kinetics of reactions helps in determining how quickly a drug will act in the body and how long it will remain effective. This information is crucial for dosage and formulation.
- Illustrative Explanation: Imagine a time-release capsule (the drug). The rate at which the capsule dissolves (reaction kinetics) determines how quickly the medication enters the bloodstream (the effect). Just as the timing of the release is critical for effectiveness, understanding kinetics is vital in pharmaceuticals.
2. Environmental Chemistry
Chemical kinetics plays a significant role in understanding pollutant degradation and the rates of chemical reactions in the environment. This knowledge helps in designing effective remediation strategies.
- Illustrative Explanation: Think of a polluted river (the environment). The rate at which contaminants break down (reaction kinetics) determines how quickly the river can recover (remediation). Just as faster breakdown leads to a cleaner river, understanding kinetics aids in environmental protection.
3. Industrial Processes
In industrial chemistry, optimizing reaction rates is essential for maximizing yield and efficiency. Kinetics helps in designing reactors and controlling reaction conditions.
- Illustrative Explanation: Imagine a factory assembly line (the industrial process). The speed at which products are assembled (reaction rate) affects overall production efficiency. Just as optimizing the assembly line increases output, understanding kinetics enhances industrial processes.
Conclusion
In conclusion, chemical kinetics is a vital field of study that explores the rates of chemical reactions and the factors influencing these rates. Understanding the fundamental concepts, reaction rates, mechanisms, and applications of chemical kinetics is essential for predicting and controlling chemical processes in various fields. As we continue to advance our knowledge in this area, chemical kinetics will remain a cornerstone of research and innovation, enabling us to develop new technologies, improve industrial processes, and address environmental challenges. By fostering awareness of these principles, we can better harness the power of chemical reactions to benefit society and the planet.