Electrochemistry is the branch of chemistry that deals with the relationship between electrical energy and chemical change. It encompasses the study of chemical reactions that involve the transfer of electrons, which can occur in various systems, including batteries, fuel cells, electrolysis, and corrosion processes. Understanding electrochemistry is essential for developing new energy technologies, improving industrial processes, and addressing environmental challenges. This article aims to provide an exhaustive overview of electrochemistry, detailing its key concepts, principles, processes, and applications, along with illustrative explanations of each concept.
Understanding Electrochemistry
1. Definition of Electrochemistry
Electrochemistry is defined as the study of chemical processes that involve the movement of electrons, which can be harnessed to produce electrical energy or drive chemical reactions. This field combines principles from both chemistry and physics to explore how chemical reactions can be used to generate electricity and how electrical energy can induce chemical changes.
- Illustrative Explanation: Imagine a water wheel (the electrochemical system) that generates power as water flows over it (the chemical reaction). The movement of water (the flow of electrons) turns the wheel, producing energy (electricity). Just as the water wheel converts kinetic energy into mechanical energy, electrochemistry converts chemical energy into electrical energy and vice versa.
Key Concepts in Electrochemistry
1. Redox Reactions
Redox (reduction-oxidation) reactions are fundamental to electrochemistry. These reactions involve the transfer of electrons between two species, where one species is oxidized (loses electrons) and the other is reduced (gains electrons).
- Oxidation: The process of losing electrons. For example, when zinc (Zn) reacts with copper sulfate (CuSO₄), zinc is oxidized to zinc ions (Zn²⁺).
- Reduction: The process of gaining electrons. In the same reaction, copper ions (Cu²⁺) are reduced to copper metal (Cu).
- Illustrative Explanation: Think of a game of catch (the redox reaction) where one player (the oxidized species) throws the ball (electrons) to another player (the reduced species). The player who throws the ball loses possession (loses electrons), while the player who catches it gains possession (gains electrons). Just as the ball changes hands, electrons are transferred between species in redox reactions.
2. Electrochemical Cells
Electrochemical cells are devices that convert chemical energy into electrical energy (galvanic cells) or electrical energy into chemical energy (electrolytic cells). They consist of two electrodes (anode and cathode) immersed in an electrolyte solution.
- Galvanic Cells: These cells generate electrical energy from spontaneous redox reactions. A common example is the Daniell cell, which consists of a zinc anode and a copper cathode.
- Electrolytic Cells: These cells require an external electrical source to drive non-spontaneous reactions. An example is the electrolysis of water to produce hydrogen and oxygen gases.
- Illustrative Explanation: Imagine a battery (the galvanic cell) that powers a flashlight. The chemical reactions inside the battery generate electricity, which lights up the bulb. Conversely, think of a charger (the electrolytic cell) that uses electricity to charge a device. Just as the battery provides power, electrochemical cells facilitate the conversion between chemical and electrical energy.
3. Electrodes
Electrodes are conductive materials that facilitate the transfer of electrons in electrochemical cells. There are two main types of electrodes:
- Anode: The electrode where oxidation occurs. It is the source of electrons in the cell.
- Cathode: The electrode where reduction occurs. It is the destination for electrons in the cell.
- Illustrative Explanation: Think of a racetrack (the electrochemical cell) with two lanes (the electrodes). The starting line (anode) is where the race begins (oxidation), and the finish line (cathode) is where the race ends (reduction). Just as runners (electrons) travel from the start to the finish, electrons move from the anode to the cathode during electrochemical reactions.
4. Electrolytes
Electrolytes are substances that dissociate into ions when dissolved in a solvent, allowing the solution to conduct electricity. In electrochemical cells, electrolytes facilitate the movement of ions between the anode and cathode, completing the circuit.
- Illustrative Explanation: Imagine a highway (the electrolyte) that allows cars (ions) to travel between two cities (the electrodes). The highway enables the flow of traffic, just as the electrolyte allows ions to move and maintain charge balance in the electrochemical cell.
Principles of Electrochemistry
1. Nernst Equation
The Nernst equation relates the cell potential (voltage) of an electrochemical cell to the concentrations of the reactants and products. It is expressed as:
![]()
Where:
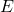 = cell potential
= cell potential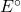 = standard cell potential
= standard cell potential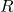 = universal gas constant
= universal gas constant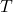 = temperature in Kelvin
= temperature in Kelvin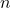 = number of moles of electrons transferred
= number of moles of electrons transferred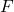 = Faraday’s constant
= Faraday’s constant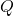 = reaction quotient
= reaction quotient- Illustrative Explanation: Think of a scale (the Nernst equation) that measures the balance of weights (concentrations of reactants and products). If one side is heavier (higher concentration), the scale tips (the cell potential changes). Just as the scale reflects the balance of weights, the Nernst equation shows how concentrations affect the voltage of an electrochemical cell.
2. Standard Electrode Potentials
Standard electrode potentials (E°) are measured under standard conditions (1 M concentration, 1 atm pressure, and 25°C) and indicate the tendency of a species to be reduced. A higher E° value means a greater tendency to gain electrons.
- Illustrative Explanation: Imagine a popularity contest (the standard electrode potentials) where candidates (chemical species) are ranked based on how many votes (electrons) they can attract. The more popular candidates (higher E° values) are more likely to win (be reduced). Just as popularity influences election outcomes, standard electrode potentials determine the likelihood of reduction in electrochemical reactions.
Factors Affecting Electrochemical Reactions
Several factors can influence the rate and efficiency of electrochemical reactions. Understanding these factors is crucial for optimizing electrochemical processes.
1. Concentration of Reactants
The concentration of reactants in an electrochemical cell affects the rate of reaction. Higher concentrations of reactants lead to increased collision frequency and, consequently, a higher reaction rate.
- Illustrative Explanation: Think of a crowded party (the electrochemical cell) where guests (reactants) are mingling. The more guests there are, the more likely they are to interact and form connections (react). Just as a crowded party leads to more interactions, higher concentrations of reactants enhance electrochemical reactions.
2. Temperature
Temperature affects the kinetic energy of molecules, influencing the rate of electrochemical reactions. Generally, increasing temperature increases reaction rates due to higher molecular motion.
- Illustrative Explanation: Imagine a pot of water on a stove. As the heat increases (temperature), the water molecules move faster and collide more often, leading to quicker boiling (faster reactions). Just as heating speeds up the boiling process, higher temperatures accelerate electrochemical reactions.
3. Surface Area of Electrodes
The surface area of electrodes can significantly impact the rate of electrochemical reactions. Larger surface areas provide more active sites for reactions to occur, enhancing the overall reaction rate.
- Illustrative Explanation: Think of a large sponge (the electrode) versus a small sponge. The larger sponge has more surface area for water (the reactant) to cling to, allowing it to absorb more water (react faster). Just as a larger sponge can hold more water, electrodes with greater surface area facilitate more efficient electrochemical reactions.
4. Nature of the Electrolyte
The type of electrolyte used in an electrochemical cell can influence the conductivity and overall performance of the cell. Different electrolytes can affect ion mobility and reaction kinetics.
- Illustrative Explanation: Imagine a race track (the electrolyte) where different types of vehicles (ions) are racing. Some vehicles (ions) are faster and more agile than others, affecting how quickly they can complete the race (the reaction). Just as the type of vehicle influences race outcomes, the nature of the electrolyte impacts the efficiency of electrochemical reactions.
Applications of Electrochemistry
Electrochemistry has numerous applications across various fields, including energy storage, corrosion prevention, and analytical chemistry. Understanding the principles of electrochemistry allows scientists and engineers to optimize processes for desired outcomes.
1. Batteries and Energy Storage
Electrochemistry is fundamental to the development of batteries, which store and release electrical energy through redox reactions. Common types of batteries include lithium-ion, lead-acid, and nickel-cadmium batteries.
- Illustrative Explanation: Think of a battery as a reservoir (the electrochemical cell) that stores water (electrical energy) until it is needed. When you turn on a light (use the battery), the water flows out to power the bulb. Just as the reservoir provides water on demand, batteries release stored energy when required.
2. Fuel Cells
Fuel cells convert chemical energy directly into electrical energy through electrochemical reactions. They are used in various applications, including transportation and stationary power generation.
- Illustrative Explanation: Imagine a fuel cell as a power plant (the electrochemical cell) that generates electricity from fuel (the reactants). As the fuel is consumed, it produces electricity to power homes and vehicles. Just as a power plant converts fuel into energy, fuel cells harness chemical reactions to generate electricity.
3. Corrosion Prevention
Electrochemistry plays a crucial role in understanding and preventing corrosion, which is the deterioration of materials due to electrochemical reactions. Techniques such as cathodic protection and coatings are used to mitigate corrosion.
- Illustrative Explanation: Think of a metal structure (the material) exposed to the elements (the environment). Without protection, the metal can corrode (deteriorate) over time. Applying a protective coating (cathodic protection) is like putting an umbrella over the structure to shield it from rain (corrosive agents). Just as the umbrella prevents water damage, corrosion prevention techniques protect materials from electrochemical degradation.
4. Analytical Chemistry
Electrochemical methods are widely used in analytical chemistry for the detection and quantification of substances. Techniques such as potentiometry and voltammetry rely on electrochemical principles to analyze samples.
- Illustrative Explanation: Imagine a detective (the analytical chemist) using a magnifying glass (electrochemical method) to examine clues (substances) at a crime scene. The magnifying glass helps the detective see details that are not visible to the naked eye (detect and quantify substances). Just as the detective uses tools to gather evidence, electrochemical methods provide valuable insights into chemical compositions.
Conclusion
In conclusion, electrochemistry is a vital field that explores the relationship between electrical energy and chemical change. By understanding the key concepts, principles, and applications of electrochemistry, researchers and engineers can develop new technologies, improve industrial processes, and address environmental challenges. As we continue to advance our knowledge in this area, electrochemistry will remain a cornerstone of research and innovation, enabling us to harness the power of chemical reactions for practical applications. By fostering awareness of these principles, we can better utilize electrochemistry to benefit society and the planet.