Silver carbonate is an inorganic compound with the chemical formula ![]() . It is a white, crystalline solid that is relatively unstable and can decompose upon heating. Below, we will explore the chemical structure, properties, and relevant aspects of silver carbonate in detail.
. It is a white, crystalline solid that is relatively unstable and can decompose upon heating. Below, we will explore the chemical structure, properties, and relevant aspects of silver carbonate in detail.
Silver carbonate (![]() ) is an important inorganic compound with a unique chemical structure characterized by its ionic bonding between silver ions and the carbonate ion. Its physical properties, such as solubility and thermal stability, along with its chemical reactivity, make it valuable in various applications, particularly in the synthesis of silver compounds and in analytical chemistry. Understanding the characteristics and properties of silver carbonate is essential for its effective use in scientific and industrial contexts.
) is an important inorganic compound with a unique chemical structure characterized by its ionic bonding between silver ions and the carbonate ion. Its physical properties, such as solubility and thermal stability, along with its chemical reactivity, make it valuable in various applications, particularly in the synthesis of silver compounds and in analytical chemistry. Understanding the characteristics and properties of silver carbonate is essential for its effective use in scientific and industrial contexts.
Chemical Structure and Properties Silver Carbonate
1. Chemical Structure
a. Molecular Formula
The molecular formula of silver carbonate is ![]() , indicating that each formula unit consists of two silver (Ag) ions and one carbonate (CO₃²⁻) ion.
, indicating that each formula unit consists of two silver (Ag) ions and one carbonate (CO₃²⁻) ion.
b. Structural Representation
- Carbonate Ion: The carbonate ion (
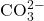 ) has a trigonal planar structure, where the carbon atom is centrally located and bonded to three oxygen atoms. The bond angles between the oxygen atoms are approximately 120 degrees. The carbonate ion carries a -2 charge, which is balanced by the two +1 charges from the two silver ions.
) has a trigonal planar structure, where the carbon atom is centrally located and bonded to three oxygen atoms. The bond angles between the oxygen atoms are approximately 120 degrees. The carbonate ion carries a -2 charge, which is balanced by the two +1 charges from the two silver ions. - Coordination: In silver carbonate, the silver ions are coordinated to the carbonate ion. The silver ions do not form covalent bonds with the carbonate but rather interact through ionic bonding. The structure can be represented as follows:
![]()
This representation highlights the ionic nature of the compound, where the silver ions are associated with the negatively charged carbonate ion.
2. Physical Properties
a. Appearance
- Color and Form: Silver carbonate typically appears as a white to pale yellow crystalline powder. The color can vary slightly depending on the purity and the presence of impurities.
b. Solubility
- Water Solubility: Silver carbonate is sparingly soluble in water. Its solubility is influenced by the presence of other ions in solution, and it can precipitate out of solution under certain conditions.
- Solubility in Other Solvents: It is more soluble in ammonia and other polar solvents, which can help dissolve the compound for various applications.
c. Melting Point and Decomposition
- Melting Point: Silver carbonate has a melting point of approximately 200 °C (392 °F). However, it does not melt in the traditional sense; instead, it decomposes upon heating.
- Decomposition: Upon heating, silver carbonate decomposes to form silver oxide (
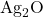 ), carbon dioxide (
), carbon dioxide (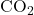 ), and oxygen (
), and oxygen (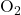 ). The decomposition reaction can be represented as follows:
). The decomposition reaction can be represented as follows:
![]()
This reaction is significant in applications where silver oxide is desired.
3. Chemical Properties
a. Reactivity
- Acid-Base Reactions: Silver carbonate can react with acids to produce carbon dioxide gas and the corresponding silver salt. For example, when silver carbonate reacts with hydrochloric acid, the reaction can be represented as:
![]()
This reaction illustrates the compound’s ability to release carbon dioxide when reacting with acids.
- Formation of Silver Compounds: Silver carbonate can be used to synthesize various silver compounds, including silver halides, which are important in photographic processes and other applications.
b. Stability
- Thermal Stability: Silver carbonate is relatively unstable and can decompose upon exposure to heat. This instability limits its use in high-temperature applications.
- Light Sensitivity: Like many silver compounds, silver carbonate can be sensitive to light, which may lead to photodecomposition under certain conditions.
Synthesis of Silver Carbonate
Silver carbonate can be synthesized through various methods, with the most common involving the reaction of silver nitrate (AgNO₃) with sodium carbonate (Na₂CO₃) or potassium carbonate (K₂CO₃).
1. Reaction with Sodium Carbonate
The reaction can be represented by the following balanced chemical equation:
![]()
In this reaction, silver nitrate reacts with sodium carbonate to produce silver carbonate and sodium nitrate.
Illustrative Example: Imagine a cooking recipe where you combine two ingredients (silver nitrate and sodium carbonate) to create a new dish (silver carbonate). The end product is a result of the careful combination of the initial ingredients.
2. Precipitation Method
Another method for synthesizing silver carbonate involves the precipitation reaction, where silver ions are mixed with carbonate ions in solution. This can be done by adding a soluble carbonate salt to a solution containing silver ions.
Illustrative Example: Picture a painter mixing two colors on a palette. When the colors (silver ions and carbonate ions) are combined, they create a new color (silver carbonate) that is distinct from the original colors.
Reactions of Silver Carbonate
Silver carbonate can undergo various chemical reactions, particularly when exposed to heat or acids. Understanding these reactions is crucial for its applications in different fields.
1. Decomposition Reaction
When heated, silver carbonate decomposes into silver oxide and carbon dioxide:
![]()
This reaction is significant because it illustrates the instability of silver carbonate at elevated temperatures.
Illustrative Example: Think of silver carbonate as a balloon filled with air. When you apply heat (like squeezing the balloon), it pops and releases the air (carbon dioxide), leaving behind a smaller, more stable object (silver oxide).
2. Reaction with Acids
Silver carbonate reacts with acids to produce carbon dioxide gas, water, and a silver salt. For example, when silver carbonate reacts with hydrochloric acid (HCl), the reaction can be represented as follows:
![]()
In this reaction, silver chloride (AgCl) is formed along with water and carbon dioxide.
Illustrative Example: Imagine a fizzy drink where the bubbles represent carbon dioxide gas being released. When silver carbonate meets an acid, it produces a similar effervescence as the gas escapes.
Applications of Silver Carbonate
Silver carbonate has several important applications across various fields, including chemistry, medicine, and materials science.
1. Synthesis of Silver Compounds
Silver carbonate is often used as a precursor for the synthesis of other silver compounds, such as silver chloride and silver oxide. These compounds have various applications, including in photography, electronics, and antimicrobial treatments.
Illustrative Example: Think of silver carbonate as a raw material in a factory. Just as raw materials are transformed into finished products, silver carbonate is converted into other valuable silver compounds.
2. Antimicrobial Agent
Silver compounds, including silver carbonate, are known for their antimicrobial properties. Silver ions can inhibit the growth of bacteria, fungi, and viruses, making silver carbonate useful in medical applications, such as wound dressings and coatings for medical devices.
Illustrative Example: Imagine a shield protecting a castle from invaders. Silver carbonate acts like this shield, providing a barrier against harmful microorganisms.
3. Catalyst in Organic Reactions
Silver carbonate can serve as a catalyst in various organic reactions, particularly in the synthesis of organic compounds. Its ability to facilitate reactions without being consumed makes it valuable in organic chemistry.
Illustrative Example: Picture a traffic light directing cars at an intersection. The traffic light (silver carbonate) helps manage the flow of traffic (chemical reactions) without being part of the cars themselves.
4. Research and Analytical Chemistry
In research and analytical chemistry, silver carbonate is used in various experiments and analyses, including the determination of carbonate content in samples and the study of silver ion behavior in different environments.
Illustrative Example: Think of a detective using tools to solve a mystery. Silver carbonate serves as a tool for chemists to uncover information about chemical compositions and reactions.
Safety and Handling
While silver carbonate is generally considered safe to handle, it is essential to follow proper safety protocols when working with this compound. It can cause irritation to the skin, eyes, and respiratory system if inhaled or ingested. Therefore, it is crucial to use personal protective equipment (PPE), such as gloves and goggles, and to work in a well-ventilated area.
Illustrative Example: Imagine a chef in a kitchen wearing an apron and gloves while handling sharp knives and hot pans. Just as the chef takes precautions to ensure safety, chemists must also be cautious when working with chemicals like silver carbonate.
Conclusion
Silver carbonate is a versatile inorganic compound with significant importance in various fields, including chemistry, medicine, and materials science. Its unique properties, synthesis methods, and reactions make it a valuable substance for researchers and industries alike. By understanding the characteristics and applications of silver carbonate, we can appreciate its role in advancing scientific knowledge and improving technological innovations. Whether used as a precursor for other silver compounds, an antimicrobial agent, or a catalyst in organic reactions, silver carbonate continues to be an essential component in the world of chemistry.