Electron configuration is a fundamental concept in chemistry and physics that describes the distribution of electrons in an atom or ion. Understanding electron configuration is essential for grasping the behavior of elements, their chemical properties, and how they interact with one another. This article will explore the principles of electron configuration, the rules governing it, the significance of electron configurations in the periodic table, and illustrative explanations to clarify each concept.
What is Electron Configuration?
Definition
Electron configuration refers to the arrangement of electrons in the atomic orbitals of an atom. It provides a detailed description of how electrons are distributed among the various energy levels and sublevels, which ultimately influences the chemical behavior of the element.
- Illustrative Explanation: Imagine a multi-story building where each floor represents an energy level, and each room on a floor represents an orbital. Just as people occupy different rooms on different floors, electrons occupy various orbitals at different energy levels within an atom.
Key Concepts
1. Energy Levels: Electrons are arranged in energy levels (or shells) around the nucleus of an atom. These levels are designated by principal quantum numbers (n), where n = 1, 2, 3, etc. The higher the value of n, the further the energy level is from the nucleus.
2. Sublevels and Orbitals: Each energy level contains sublevels (s, p, d, f) that have different shapes and capacities for holding electrons. Each sublevel consists of one or more orbitals, which are regions in space where electrons are likely to be found.
3. Pauli Exclusion Principle: This principle states that no two electrons in an atom can have the same set of four quantum numbers. In simpler terms, an orbital can hold a maximum of two electrons, and they must have opposite spins.
4. Hund’s Rule: When electrons occupy orbitals of the same energy (degenerate orbitals), they will fill each orbital singly before pairing up. This minimizes electron-electron repulsion and leads to a more stable configuration.
5. Aufbau Principle: This principle states that electrons fill orbitals starting from the lowest energy level to the highest. The order of filling is determined by the relative energies of the orbitals.
The Electron Configuration Notation
Electron configurations are typically written using a notation that indicates the energy levels, sublevels, and the number of electrons in each sublevel. The general format is:
![]()
For example, the electron configuration of carbon (atomic number 6) is written as:
![]()
This notation indicates that carbon has:
- 2 electrons in the 1s sublevel
- 2 electrons in the 2s sublevel
- 2 electrons in the 2p sublevel
Illustrative Explanation
Think of a library where books are organized by sections (energy levels) and shelves (sublevels). Each shelf can hold a certain number of books (electrons). The electron configuration tells you how many books are on each shelf, helping you understand the organization of the library (the atom).
Steps to Determine Electron Configuration
To determine the electron configuration of an element, follow these steps:
1. Identify the Atomic Number
The atomic number of an element indicates the number of protons in its nucleus, which is equal to the number of electrons in a neutral atom.
- Illustrative Explanation: Imagine counting the number of apples in a basket. Just as the number of apples represents the total quantity, the atomic number represents the total number of electrons in a neutral atom.
2. Use the Aufbau Principle
Start filling the orbitals according to the Aufbau principle, beginning with the lowest energy level and moving to higher levels. Follow the order of filling based on the energy levels and sublevels.
- Illustrative Explanation: Think of filling a glass with water. Just as you pour water into the glass starting from the bottom, you fill the orbitals starting from the lowest energy level.
3. Apply the Pauli Exclusion Principle and Hund’s Rule
Ensure that no orbital contains more than two electrons with opposite spins, and fill degenerate orbitals singly before pairing up.
- Illustrative Explanation: Imagine a game of musical chairs where each chair (orbital) can only hold two players (electrons). Just as players fill chairs one by one before sharing, electrons fill orbitals according to these rules.
4. Write the Configuration
Once all electrons are placed in their respective orbitals, write the electron configuration using the appropriate notation.
- Illustrative Explanation: Consider writing a summary of a story. Just as you summarize the main points, you write the electron configuration to summarize the arrangement of electrons in an atom.
Examples of Electron Configurations
1. Hydrogen (H)
- Atomic Number: 1
- Electron Configuration:
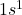
Hydrogen has one electron, which occupies the 1s orbital.
2. Oxygen (O)
- Atomic Number: 8
- Electron Configuration:
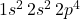
Oxygen has eight electrons, filling the 1s and 2s orbitals and partially filling the 2p orbital.
3. Iron (Fe)
- Atomic Number: 26
- Electron Configuration:
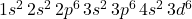
Iron has 26 electrons, filling the 1s, 2s, 2p, 3s, 3p, and 4s orbitals, and partially filling the 3d orbital.
4. Uranium (U)
- Atomic Number: 92
- Electron Configuration:
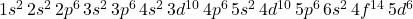
Uranium has 92 electrons, filling the lower energy levels and sublevels before reaching the higher energy levels.
Significance of Electron Configuration
Understanding electron configuration is crucial for several reasons:
1. Chemical Properties
The electron configuration of an element determines its chemical properties, including reactivity, ionization energy, and electronegativity. Elements with similar configurations often exhibit similar chemical behavior.
- Illustrative Explanation: Think of a group of friends with similar interests. Just as friends with similar hobbies tend to enjoy the same activities, elements with similar electron configurations often react in similar ways.
2. Periodic Table Organization
The periodic table is organized based on the electron configurations of elements. Elements in the same group (column) have similar valence electron configurations, which influences their chemical properties.
- Illustrative Explanation: Imagine a family reunion where relatives share similar traits. Just as family members with common characteristics gather together, elements with similar electron configurations are grouped in the periodic table.
3. Predicting Bonding Behavior
Electron configuration helps predict how atoms will bond with one another. Atoms tend to form bonds to achieve a stable electron configuration, often resembling that of noble gases.
- Illustrative Explanation: Consider a puzzle where each piece must fit together. Just as puzzle pieces connect to form a complete picture, atoms bond to achieve stable electron configurations.
4. Understanding Spectroscopy
Electron configurations are essential for understanding atomic spectra and the behavior of electrons when they absorb or emit energy. This knowledge is crucial in fields such as astrophysics and analytical chemistry.
- Illustrative Explanation: Think of a musical instrument producing different notes. Just as the instrument’s design determines the sounds it can make, the electron configuration influences the energy levels and transitions that produce spectral lines.
Conclusion
Electron configuration is a fundamental concept that provides insight into the arrangement of electrons in atoms and their chemical behavior. By understanding the principles, notation, and significance of electron configurations, we can better appreciate the underlying mechanisms that govern chemical reactions and the properties of elements. Whether predicting reactivity, organizing the periodic table, or exploring atomic spectra, electron configuration remains a cornerstone of modern chemistry and physics. As we continue to study and explore this concept, we unlock new possibilities for innovation and discovery in the scientific world.