Colligative properties are physical properties of solutions that depend primarily on the number of solute particles in a given quantity of solvent, rather than the identity or nature of the solute itself. These properties are crucial in various scientific fields, including chemistry, biology, and environmental science, as they provide insights into the behavior of solutions and the interactions between solute and solvent molecules. This comprehensive overview will explore the definition of colligative properties, the main types, the underlying principles, methods of measurement, and their applications in various contexts.
1. Definition of Colligative Properties
Colligative properties are defined as properties of solutions that depend on the ratio of the number of solute particles to the number of solvent molecules in a solution. These properties are independent of the chemical identity of the solute, meaning that the same number of particles will produce similar effects regardless of the type of solute. The primary colligative properties include:
- Vapor Pressure Lowering
- Boiling Point Elevation
- Freezing Point Depression
- Osmotic Pressure
2. Main Types of Colligative Properties
A. Vapor Pressure Lowering:
When a non-volatile solute is added to a solvent, the vapor pressure of the solvent decreases. This occurs because the presence of solute particles reduces the number of solvent molecules at the surface that can escape into the vapor phase. Raoult’s Law describes this phenomenon, stating that the vapor pressure of a solvent in a solution (P_solution) is equal to the product of the mole fraction of the solvent (X_solvent) and the vapor pressure of the pure solvent (P°_solvent):
![]()
B. Boiling Point Elevation:
The addition of a solute to a solvent raises the boiling point of the solution compared to that of the pure solvent. This elevation in boiling point can be quantified using the formula:
![]()
Where:
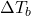 = change in boiling point
= change in boiling point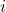 = van ‘t Hoff factor (number of particles the solute dissociates into)
= van ‘t Hoff factor (number of particles the solute dissociates into)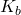 = ebullioscopic constant of the solvent
= ebullioscopic constant of the solvent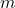 = molality of the solution
= molality of the solution
C. Freezing Point Depression:
Conversely, the presence of a solute lowers the freezing point of a solution compared to that of the pure solvent. This depression in freezing point can be expressed as:
![]()
Where:
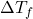 = change in freezing point
= change in freezing point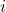 = van ‘t Hoff factor
= van ‘t Hoff factor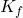 = cryoscopic constant of the solvent
= cryoscopic constant of the solvent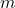 = molality of the solution
= molality of the solution
D. Osmotic Pressure:
Osmotic pressure is the pressure required to prevent the flow of solvent into a solution through a semipermeable membrane. It is directly proportional to the concentration of solute particles in the solution and can be calculated using the formula:
![]()
Where:
 = osmotic pressure
= osmotic pressure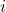 = van ‘t Hoff factor
= van ‘t Hoff factor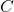 = molar concentration of the solution
= molar concentration of the solution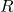 = universal gas constant (0.0821 L·atm/(K·mol))
= universal gas constant (0.0821 L·atm/(K·mol))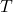 = absolute temperature in Kelvin
= absolute temperature in Kelvin
3. Underlying Principles of Colligative Properties
The underlying principles of colligative properties are rooted in the interactions between solute and solvent molecules. When a solute is added to a solvent, several key factors come into play:
A. Particle Concentration:
Colligative properties depend on the number of solute particles in a given volume of solvent. For example, adding more solute particles will have a more significant effect on the properties of the solution.
B. Solvent-Solute Interactions:
The interactions between solute and solvent molecules influence the physical properties of the solution. For instance, solute particles disrupt the orderly arrangement of solvent molecules, affecting vapor pressure, boiling point, and freezing point.
C. Colligative Effects and Ionic Compounds:
For ionic compounds, the van ‘t Hoff factor (i) is particularly important, as it accounts for the number of ions produced when the solute dissociates in solution. For example, sodium chloride (NaCl) dissociates into two ions (Na⁺ and Cl⁻), so its van ‘t Hoff factor is 2.
4. Measurement of Colligative Properties
Colligative properties can be measured using various experimental techniques:
A. Vapor Pressure Measurement:
Vapor pressure can be measured using a manometer or a vapor pressure osmometer. By comparing the vapor pressure of the solution to that of the pure solvent, the extent of vapor pressure lowering can be determined.
B. Boiling Point and Freezing Point Determination:
The boiling point elevation and freezing point depression can be measured using a simple laboratory setup. For boiling point elevation, the temperature at which the solution boils is recorded, while for freezing point depression, the temperature at which the solution freezes is noted.
C. Osmotic Pressure Measurement:
Osmotic pressure can be measured using an osmometer, which allows for the determination of the pressure required to prevent solvent flow across a semipermeable membrane.
5. Applications of Colligative Properties
Colligative properties have numerous applications across various fields:
A. Chemistry and Chemical Engineering:
Understanding colligative properties is essential for designing chemical processes, such as distillation and crystallization, where the manipulation of boiling and freezing points is crucial.
B. Biology and Medicine:
Colligative properties play a significant role in biological systems. For example, osmotic pressure is critical in maintaining cell integrity and function. In medicine, colligative properties are used in formulating intravenous solutions and understanding drug solubility.
C. Environmental Science:
Colligative properties are important in environmental science, particularly in understanding the behavior of pollutants in water bodies. The freezing point depression of saline water, for instance, affects the survival of aquatic organisms in cold environments.
D. Food Science:
In food science, colligative properties are utilized in food preservation techniques, such as salting and sugaring, which lower the freezing point of water and inhibit microbial growth.
6. Conclusion
In conclusion, colligative properties are essential characteristics of solutions that depend on the number of solute particles rather than their identity. These properties, including vapor pressure lowering, boiling point elevation, freezing point depression, and osmotic pressure, have significant implications in various scientific fields. Understanding colligative properties allows chemists, biologists, and engineers to predict and manipulate the behavior of solutions in a wide range of applications, from industrial processes to biological systems. As research continues to advance, the study of colligative properties will remain a vital aspect of understanding solution chemistry and its practical applications in everyday life.