The p-block elements are a group of elements found in groups 13 to 18 of the periodic table. These elements are characterized by the presence of their outermost electrons in p orbitals. The p-block encompasses a diverse range of elements, including metals, nonmetals, and metalloids, each exhibiting unique physical and chemical properties. This comprehensive overview will explore the classification of p-block elements, their electronic configuration, properties, trends, and applications in various fields.
1. Classification of p-Block Elements
The p-block elements can be divided into six groups, each containing distinct elements with varying properties:
A. Group 13 (III-A):
- Elements: Boron (B), Aluminum (Al), Gallium (Ga), Indium (In), Thallium (Tl), and Nihonium (Nh).
- Characteristics: This group contains both nonmetals (boron) and metals (aluminum and others). Boron is a metalloid, while the rest are metals. They typically exhibit +3 oxidation states, although some can show +1 oxidation states.
B. Group 14 (IV-A):
- Elements: Carbon (C), Silicon (Si), Germanium (Ge), Tin (Sn), Lead (Pb), and Flerovium (Fl).
- Characteristics: This group includes nonmetals (carbon), metalloids (silicon and germanium), and metals (tin and lead). The oxidation states can vary from -4 to +4, with carbon being the most versatile in forming covalent compounds.
C. Group 15 (V-A):
- Elements: Nitrogen (N), Phosphorus (P), Arsenic (As), Antimony (Sb), Bismuth (Bi), and Moscovium (Mc).
- Characteristics: This group contains nonmetals (nitrogen and phosphorus), metalloids (arsenic and antimony), and a metal (bismuth). The oxidation states range from -3 to +5, with nitrogen being notable for its ability to form multiple covalent bonds.
D. Group 16 (VI-A):
- Elements: Oxygen (O), Sulfur (S), Selenium (Se), Tellurium (Te), Polonium (Po), and Livermorium (Lv).
- Characteristics: This group includes nonmetals (oxygen and sulfur), metalloids (selenium and tellurium), and a metal (polonium). The oxidation states typically range from -2 to +6, with oxygen being essential for respiration and combustion.
E. Group 17 (VII-A):
- Elements: Fluorine (F), Chlorine (Cl), Bromine (Br), Iodine (I), Astatine (At), and Tennessine (Ts).
- Characteristics: Known as the halogens, these elements are highly reactive nonmetals. They typically exhibit -1 oxidation states but can also show positive oxidation states in certain compounds.
F. Group 18 (VIII-A):
- Elements: Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe), and Radon (Rn).
- Characteristics: The noble gases are characterized by their full valence shell, making them largely inert and nonreactive under standard conditions. They have oxidation states of 0 and are used in various applications due to their stability.
2. Electronic Configuration
The general electronic configuration of p-block elements can be expressed as:
![]()
Where:
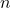 represents the principal quantum number corresponding to the period of the element.
represents the principal quantum number corresponding to the period of the element.- The outermost p subshell can hold a maximum of six electrons, leading to the filling of p orbitals.
For example:
- Boron (B): [He] 2s² 2p¹
- Carbon (C): [He] 2s² 2p²
- Nitrogen (N): [He] 2s² 2p³
- Oxygen (O): [He] 2s² 2p⁴
- Fluorine (F): [He] 2s² 2p⁵
- Neon (Ne): [He] 2s² 2p⁶
3. Properties of p-Block Elements
The p-block elements exhibit a wide range of physical and chemical properties:
A. Physical Properties:
- Metals: Generally have high melting and boiling points, good electrical and thermal conductivity, and malleability. Aluminum is a notable metal in this block.
- Nonmetals: Typically have lower melting and boiling points, are poor conductors of heat and electricity, and are often brittle in solid form. Oxygen and sulfur are examples of nonmetals.
- Metalloids: Exhibit properties intermediate between metals and nonmetals. Silicon and germanium are commonly used in semiconductor technology.
B. Chemical Properties:
- Reactivity: The reactivity of p-block elements varies significantly. For example, halogens are highly reactive, while noble gases are largely inert.
- Oxidation States: p-block elements can exhibit multiple oxidation states, allowing them to form a variety of compounds. For instance, nitrogen can exist in oxidation states ranging from -3 to +5.
- Acid-Base Behavior: Many p-block elements form acidic or basic oxides. For example, sulfur dioxide (SO₂) is an acidic oxide, while magnesium oxide (MgO) is basic.
4. Trends in p-Block Elements
Several trends can be observed within the p-block elements:
A. Atomic Size:
Atomic size generally increases down a group due to the addition of electron shells. However, there is a decrease in atomic size across a period from left to right due to increased nuclear charge, which pulls electrons closer to the nucleus.
B. Ionization Energy:
Ionization energy tends to decrease down a group as the outermost electrons are farther from the nucleus and experience greater shielding. Conversely, ionization energy increases across a period due to increased nuclear charge.
C. Electronegativity:
Electronegativity generally increases across a period and decreases down a group. Nonmetals, particularly in group 16 and 17, exhibit high electronegativity, while metals in group 13 and 14 have lower electronegativities.
D. Reactivity:
Reactivity varies widely among p-block elements. For example, alkali metals (not in the p-block) are highly reactive, while noble gases are inert. Halogens are very reactive nonmetals, while heavier p-block elements may exhibit less reactivity.
5. Applications of p-Block Elements
p-Block elements have numerous applications across various fields:
A. Electronics:
Silicon (Si) and germanium (Ge) are widely used in semiconductor technology, forming the basis of modern electronics, including transistors and diodes.
B. Materials Science:
Aluminum (Al) is used extensively in construction, transportation, and packaging due to its lightweight and corrosion-resistant properties.
C. Chemistry:
Boron (B) is used in glass and ceramics, while phosphorus (P) is essential in fertilizers and biological systems. Sulfur (S) is used in the production of sulfuric acid, one of the most widely used industrial chemicals.
D. Medicine:
Certain p-block elements, such as iodine (I), are used in medical imaging and antiseptics. Noble gases like xenon (Xe) are used in specialized lighting and anesthesia.
E. Environmental Science:
p-Block elements play a role in environmental chemistry, such as the study of pollutants and their effects on ecosystems. For example, nitrogen oxides (NOx) are significant contributors to air pollution.
6. Conclusion
In conclusion, p-block elements are a diverse group of elements that exhibit a wide range of physical and chemical properties. Their classification into groups 13 to 18 of the periodic table highlights the variety of behaviors and applications associated with these elements. Understanding the trends, properties, and applications of p-block elements is essential for advancing knowledge in chemistry, materials science, electronics, and environmental science. As research continues to evolve, the study of p-block elements will remain a vital aspect of scientific inquiry, contributing to innovations and advancements in various fields.