Coulomb’s Law is a fundamental principle in electrostatics that describes the force between two charged objects. Named after the French physicist Charles-Augustin de Coulomb, who formulated the law in the 18th century, it provides a quantitative description of the electrostatic interaction between charged particles. This comprehensive article will delve into the definition, mathematical formulation, characteristics, implications, applications, and significance of Coulomb’s Law, providing a thorough overview of this essential topic.
Definition of Coulomb’s Law
Coulomb’s Law states that the magnitude of the electrostatic force ![]() between two point charges is directly proportional to the product of the magnitudes of the charges and inversely proportional to the square of the distance
between two point charges is directly proportional to the product of the magnitudes of the charges and inversely proportional to the square of the distance ![]() between them. The law can be expressed mathematically as:
between them. The law can be expressed mathematically as:
![]()
where:
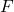 is the magnitude of the electrostatic force between the charges,
is the magnitude of the electrostatic force between the charges,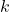 is Coulomb’s constant, approximately equal to
is Coulomb’s constant, approximately equal to 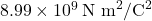 ,
,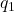 and
and 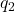 are the magnitudes of the two point charges,
are the magnitudes of the two point charges, is the distance between the centers of the two charges.
is the distance between the centers of the two charges.
Characteristics of Coulomb’s Law
Coulomb’s Law exhibits several key characteristics:
1. Inverse Square Law: The force between two charges decreases with the square of the distance between them. This means that if the distance is doubled, the force becomes one-fourth as strong.
2. Direct Proportionality: The force is directly proportional to the product of the magnitudes of the charges. If either charge is increased, the force between them increases proportionally.
3. Vector Nature: Coulomb’s Law describes a vector force, meaning it has both magnitude and direction. The direction of the force depends on the nature of the charges:
- Like charges (both positive or both negative) repel each other.
- Opposite charges (one positive and one negative) attract each other.
4. Superposition Principle: The net electrostatic force acting on a charge due to multiple other charges can be found by vectorially adding the individual forces exerted by each charge. This principle allows for the analysis of complex charge configurations.
Mathematical Formulation of Coulomb’s Law
The mathematical formulation of Coulomb’s Law can be expressed in vector form to account for direction:
![]()
where:
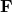 is the vector force acting on charge
is the vector force acting on charge 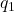 due to charge
due to charge 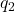 ,
,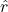 is the unit vector pointing from charge
is the unit vector pointing from charge 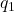 to charge
to charge 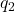 .
.
Implications of Coulomb’s Law
Coulomb’s Law has several important implications in the study of electrostatics:
1. Electric Field: Coulomb’s Law is foundational for understanding electric fields. The electric field ![]() created by a point charge
created by a point charge ![]() at a distance
at a distance ![]() is given by:
is given by:
![]()
The electric field describes the force per unit charge experienced by a test charge placed in the field.
2. Potential Energy: The potential energy ![]() associated with two point charges can be derived from Coulomb’s Law. It is given by:
associated with two point charges can be derived from Coulomb’s Law. It is given by:
![]()
This potential energy is crucial for understanding the stability of charge configurations and the work done in moving charges.
3. Charge Conservation: Coulomb’s Law supports the principle of charge conservation, which states that the total electric charge in an isolated system remains constant. This principle is fundamental in electrostatics and electrical engineering.
Applications of Coulomb’s Law
Coulomb’s Law has numerous applications across various fields, including:
1. Electrostatics: Coulomb’s Law is essential for calculating the forces between charged objects in electrostatic systems, such as charged spheres, plates, and conductors.
2. Electronics: Understanding the interactions between charged particles is crucial in the design and operation of electronic components, such as capacitors, transistors, and integrated circuits.
3. Chemical Bonding: Coulomb’s Law plays a significant role in explaining ionic and covalent bonding in chemistry. The attraction between positively charged nuclei and negatively charged electrons is governed by electrostatic forces.
4. Physics and Engineering: Coulomb’s Law is foundational in fields such as physics, electrical engineering, and materials science, where it is used to analyze and design systems involving electric charges and fields.
5. Nanotechnology: In nanotechnology, Coulomb’s Law is used to understand the behavior of charged nanoparticles and their interactions, which are critical for applications in drug delivery, sensors, and materials development.
Significance of Coulomb’s Law
The significance of Coulomb’s Law extends beyond its applications:
1. Fundamental Understanding: Coulomb’s Law is one of the cornerstones of classical electromagnetism, providing a fundamental understanding of electric forces and fields. It is essential for students and professionals in physics and engineering.
2. Historical Context: The formulation of Coulomb’s Law marked a significant advancement in the understanding of electrostatics and laid the groundwork for later developments in electromagnetism, including Maxwell’s equations.
3. Research and Development: The principles derived from Coulomb’s Law continue to inform research in various fields, including materials science, nanotechnology, and quantum mechanics. Understanding electrostatic interactions is crucial for developing new materials and technologies.
4. Environmental Impact: Coulomb’s Law helps explain phenomena such as atmospheric electricity, lightning, and the behavior of charged particles in the environment. Understanding these interactions is important for addressing environmental challenges.
Conclusion
In conclusion, Coulomb’s Law is a fundamental principle in electrostatics that describes the force between two charged objects. Its mathematical formulation, characteristics, implications, applications, and significance highlight its importance in understanding electric forces and fields. Coulomb’s Law not only enhances our knowledge of electrostatics but also informs practical applications that impact technology, chemistry, and environmental science. As research continues to advance, the study of Coulomb’s Law will remain a dynamic area of exploration, driving innovations that contribute to our understanding of electric phenomena and the development of new technologies. The future of research related to Coulomb’s Law holds great promise, with ongoing developments aimed at harnessing its principles for improving energy efficiency, materials design, and electronic systems. By addressing the challenges posed by electrostatic interactions, we can enhance our ability to design and implement systems that meet the demands of modern society while promoting scientific advancement and environmental stewardship.