Water is a fundamental substance that plays a crucial role in the Earth’s ecosystems, human life, and various scientific processes. One of the key physical properties of water is its density, which significantly influences its behavior in different environments. This article will provide an in-depth examination of the density of water, covering its definition, factors affecting density, measurement techniques, implications in nature and science, and illustrative explanations to enhance understanding.
1. Definition of Density
Density is defined as the mass of a substance per unit volume. It is a measure of how much matter is packed into a given space. The formula for density (![]() ) is given by:
) is given by:
![]()
where:
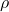 is the density,
is the density,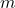 is the mass of the substance, and
is the mass of the substance, and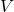 is the volume of the substance.
is the volume of the substance.
For water, the standard density at 4 degrees Celsius (the temperature at which water is most dense) is approximately 1 gram per cubic centimeter (g/cm³) or 1000 kilograms per cubic meter (kg/m³).
Illustrative Explanation: Imagine a box filled with different types of fruit. If you have a box of apples (mass) that takes up a certain amount of space (volume), the density tells you how many apples are packed into that space. If you replace some apples with watermelons (which are heavier), the density of the box changes because the mass has increased while the volume remains the same.
2. Factors Affecting the Density of Water
The density of water can be influenced by several factors, including temperature, salinity, and pressure.
2.1. Temperature
Description: The density of water changes with temperature. As water is heated, it expands, causing its density to decrease. Conversely, as water cools, it contracts, and its density increases. However, water exhibits a unique behavior: it reaches its maximum density at around 4 degrees Celsius.
Illustrative Explanation: Think of a balloon filled with air. When you heat the balloon, the air inside expands, causing the balloon to become larger and lighter (lower density). When you cool the balloon, the air contracts, making it smaller and denser. Water behaves similarly, but it has a special point (4 degrees Celsius) where it is at its densest.
2.2. Salinity
Description: The presence of dissolved salts in water increases its density. This is because the mass of the water increases due to the added salt, while the volume remains relatively constant. Seawater, for example, has a higher density than freshwater due to its salt content.
Illustrative Explanation: Imagine a glass of pure water and a glass of saltwater. If you add salt to the water, the overall weight of the glass increases (more mass), but the space it occupies doesn’t change much. As a result, the saltwater becomes denser than the pure water, just like adding more weight to a backpack makes it heavier without increasing its size.
2.3. Pressure
Description: In deep bodies of water, such as oceans, pressure increases with depth. This increase in pressure can compress water slightly, leading to a small increase in density. However, the effect of pressure on the density of water is relatively minor compared to temperature and salinity.
Illustrative Explanation: Picture a sponge submerged in water. As you push down on the sponge (increasing pressure), it compresses slightly, making it denser. Similarly, as you go deeper into the ocean, the water is subjected to greater pressure, which can slightly increase its density.
3. Measurement of Water Density
The density of water can be measured using various methods, including:
- Hydrometers: A hydrometer is a device that floats in a liquid and measures its density based on the level to which it sinks. The scale on the hydrometer indicates the density of the liquid.
- Pycnometers: A pycnometer is a specialized flask used to determine the density of liquids. It is filled with a known volume of water, and the mass is measured. The density is then calculated using the formula for density.
- Archimedes’ Principle: This principle states that the buoyant force on an object submerged in a fluid is equal to the weight of the fluid displaced by the object. By measuring the weight of an object in air and in water, the density of the water can be inferred.
Illustrative Explanation: Imagine using a measuring cup to determine how much water you have. If you fill the cup to a certain level and then weigh it, you can calculate the density of the water inside. Just like measuring ingredients for a recipe, measuring water density helps us understand its properties.
4. Implications of Water Density in Nature and Science
The density of water has significant implications in various natural processes and scientific phenomena:
- Buoyancy: The density of water determines whether objects float or sink. An object will float if its density is less than that of water and sink if its density is greater. This principle is crucial for understanding the behavior of boats, fish, and other objects in water.
- Ocean Currents: Variations in water density due to temperature and salinity create ocean currents. Denser water tends to sink, while less dense water rises, driving the movement of ocean currents that play a vital role in regulating the Earth’s climate.
- Weather Patterns: The density of water vapor in the atmosphere affects weather patterns. Warm air can hold more moisture than cold air, influencing precipitation and cloud formation.
Illustrative Explanation: Think of a swimming pool filled with different objects. A beach ball floats on the surface because it is less dense than water, while a rock sinks because it is denser. Similarly, ocean currents can be visualized as a conveyor belt, where denser water sinks and less dense water rises, creating a continuous flow that affects marine life and climate.
5. The Unique Properties of Water Density
Water exhibits several unique properties related to its density that are essential for life on Earth:
- Anomalous Expansion: Unlike most substances, water expands when it freezes, making ice less dense than liquid water. This is why ice floats on water, providing insulation for aquatic life during cold weather.
- Thermal Regulation: Water’s high specific heat capacity (the amount of heat required to change its temperature) allows it to absorb and release heat slowly. This property helps regulate temperatures in the environment and is crucial for maintaining stable climates.
- Solvent Properties: Water’s density and ability to dissolve various substances make it an excellent solvent, facilitating chemical reactions and biological processes essential for life.
Illustrative Explanation: Imagine a lake in winter. As the water cools, it becomes denser until it reaches 4 degrees Celsius. When it freezes, the ice forms on the surface, creating a protective layer that insulates the water below, allowing fish and other aquatic life to survive. This unique behavior of water is like a warm blanket that keeps the lake alive during the cold months.
Conclusion
The density of water is a fundamental property that influences a wide range of natural processes and scientific phenomena. By exploring the definitions, factors affecting density, measurement techniques, implications in nature and science, and unique properties of water density, we gain a deeper appreciation for its significance in our world. From buoyancy and ocean currents to the unique behavior of ice, the density of water plays a crucial role in sustaining life and shaping the environment. Understanding this property not only enhances our knowledge of water but also underscores its importance in various scientific and ecological contexts. As we continue to study water and its properties, we unlock new insights into the intricate systems that govern our planet.