Ions are charged particles that form when atoms gain or lose electrons. This process alters the balance between the number of protons (positively charged) and electrons (negatively charged) in an atom, resulting in a net charge. Ions are classified into two main categories: cations and anions. Understanding the differences between these two types of ions is fundamental to the study of chemistry, as they play crucial roles in chemical reactions, the formation of compounds, and the behavior of substances in various environments. This article provides a detailed overview of cations and anions, including their definitions, characteristics, formation, and illustrative explanations of each concept to enhance understanding.
Definition of Cations and Anions
What is a Cation?
A cation is a positively charged ion that forms when an atom loses one or more electrons. The loss of negatively charged electrons results in a net positive charge, as the number of protons in the nucleus remains unchanged. Cations are typically formed by metals, which have a tendency to lose electrons due to their lower electronegativity.
Example: Sodium (Na) can lose one electron to form a sodium cation (![]() ):
):
![]()
What is an Anion?
An anion is a negatively charged ion that forms when an atom gains one or more electrons. The addition of negatively charged electrons results in a net negative charge, as the number of protons remains constant. Anions are typically formed by nonmetals, which have a higher electronegativity and a greater tendency to gain electrons.
Example: Chlorine (Cl) can gain one electron to form a chloride anion (![]() ):
):
![]()
Illustrative Explanation
To visualize the difference between cations and anions, think of a balance scale. In this analogy, the protons represent weights on one side of the scale, while the electrons represent weights on the other side.
- When an atom loses electrons (forming a cation), it becomes “lighter” on the electron side, resulting in a positive charge. Imagine removing weights from the left side of the scale, causing it to tip to the right (positive charge).
- Conversely, when an atom gains electrons (forming an anion), it becomes “heavier” on the electron side, resulting in a negative charge. This is like adding weights to the left side of the scale, causing it to tip to the left (negative charge).
Characteristics of Cations and Anions
1. Charge
- Cations: Always carry a positive charge. The charge is indicated by a superscript plus sign (+) after the element symbol. For example,
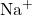 indicates a sodium cation with a +1 charge.
indicates a sodium cation with a +1 charge. - Anions: Always carry a negative charge. The charge is indicated by a superscript minus sign (−) after the element symbol. For example,
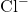 indicates a chloride anion with a −1 charge.
indicates a chloride anion with a −1 charge.
2. Formation
- Cations: Formed by the loss of electrons, typically from metals. Metals have fewer electrons in their outer shell and tend to lose them to achieve a stable electron configuration (often resembling the nearest noble gas).
- Anions: Formed by the gain of electrons, typically from nonmetals. Nonmetals have more electrons in their outer shell and tend to gain electrons to achieve a stable electron configuration.
3. Size
- Cations: Generally smaller than their parent atoms. When an atom loses electrons, the remaining electrons are drawn closer to the nucleus due to the increased positive charge, resulting in a smaller ionic radius.
- Anions: Generally larger than their parent atoms. When an atom gains electrons, the increased electron-electron repulsion causes the electron cloud to expand, resulting in a larger ionic radius.
Illustrative Explanation
Imagine a balloon representing an atom.
- When you let air out of the balloon (representing the loss of electrons), the balloon shrinks in size, similar to how a cation becomes smaller than its parent atom.
- Conversely, when you blow air into the balloon (representing the gain of electrons), the balloon expands, similar to how an anion becomes larger than its parent atom.
Examples of Cations and Anions
Common Cations
1. Sodium Ion (![]() ): Formed from sodium by losing one electron.
): Formed from sodium by losing one electron.
2. Calcium Ion (![]() ): Formed from calcium by losing two electrons.
): Formed from calcium by losing two electrons.
3. Aluminum Ion (![]() ): Formed from aluminum by losing three electrons.
): Formed from aluminum by losing three electrons.
Common Anions
1. Chloride Ion (![]() ): Formed from chlorine by gaining one electron.
): Formed from chlorine by gaining one electron.
2. Sulfide Ion (![]() ): Formed from sulfur by gaining two electrons.
): Formed from sulfur by gaining two electrons.
3. Nitrate Ion (![]() ): A polyatomic ion formed from nitrogen and oxygen, carrying a −1 charge.
): A polyatomic ion formed from nitrogen and oxygen, carrying a −1 charge.
Illustrative Explanation
Think of cations and anions as characters in a story. The cations (like sodium, calcium, and aluminum) are the heroes who lose their electrons to become stronger and more stable. The anions (like chloride, sulfide, and nitrate) are the allies who gain electrons to complete their missions and achieve stability. Together, they form ionic compounds, much like heroes and allies teaming up to achieve a common goal.
Role of Cations and Anions in Ionic Compounds
Cations and anions play a crucial role in the formation of ionic compounds. When cations and anions combine, they form ionic bonds, resulting in the creation of neutral compounds. The overall charge of the compound must be zero, which means that the total positive charge from the cations must balance the total negative charge from the anions.
Example of Ionic Compound Formation
Consider the formation of sodium chloride (table salt):
1. Formation of Cations and Anions:
- Sodium (Na) loses one electron to form a sodium cation (
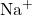 ).
). - Chlorine (Cl) gains one electron to form a chloride anion (
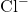 ).
).
2. Ionic Bond Formation:
- The sodium cation and chloride anion attract each other due to their opposite charges, forming the ionic compound sodium chloride (
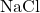 ).
).
Illustrative Explanation
Imagine a dance where cations and anions are partners. The cations (like sodium) are eager to lose their electrons (like giving away a dance move), while the anions (like chloride) are ready to gain those electrons (like receiving a dance move). When they come together, they create a beautiful dance (ionic bond) that results in a stable compound (sodium chloride).
Conclusion
In conclusion, cations and anions are fundamental concepts in chemistry that represent positively and negatively charged ions, respectively. Understanding the differences between these two types of ions, including their formation, characteristics, and roles in ionic compounds, is essential for grasping the principles of chemical bonding and reactivity. Through illustrative explanations and practical examples, we can appreciate the significance of cations and anions in the world of chemistry and their impact on the formation of compounds that are vital to life and various industrial processes. As we continue to explore the intricacies of chemical interactions, mastering the concepts of cations and anions will empower us to unlock the mysteries of matter and the principles that govern the behavior of substances in our universe.