Enthalpy change is a fundamental concept in thermodynamics that describes the heat content of a system during a chemical reaction or physical process at constant pressure. It is a crucial parameter for understanding energy changes in chemical reactions, phase transitions, and various physical processes. This article will provide a detailed examination of enthalpy change, including its definition, significance, calculation methods, types, and illustrative explanations to enhance comprehension.
1. Overview of Enthalpy Change
Definition: Enthalpy change (![]() ) is the difference in enthalpy between the products and reactants of a chemical reaction. It is expressed in joules (J) or kilojoules (kJ) and indicates whether a reaction is exothermic (releases heat) or endothermic (absorbs heat).
) is the difference in enthalpy between the products and reactants of a chemical reaction. It is expressed in joules (J) or kilojoules (kJ) and indicates whether a reaction is exothermic (releases heat) or endothermic (absorbs heat).
Illustrative Explanation: Think of enthalpy change as the energy balance in a bank account. Just as you can deposit (gain) or withdraw (lose) money, a chemical reaction can either absorb energy (endothermic) or release energy (exothermic). The enthalpy change tells you how much energy is gained or lost during the process.
2. Significance of Enthalpy Change
Enthalpy change is significant for several reasons:
A. Understanding Reaction Energetics
- Definition: Enthalpy change provides insight into the energy dynamics of a reaction, helping chemists understand whether a reaction will occur spontaneously and how much energy is involved.
- Illustrative Explanation: Imagine a roller coaster ride. The height of the coaster at the start represents the energy of the reactants, while the height at the end represents the energy of the products. The difference in height (enthalpy change) indicates how thrilling the ride (reaction) will be—whether it will be a gentle slope (endothermic) or a steep drop (exothermic).
B. Predicting Reaction Feasibility
- Definition: The sign of the enthalpy change can help predict whether a reaction will occur under certain conditions. Exothermic reactions tend to be more favorable in terms of spontaneity.
- Illustrative Explanation: Think of enthalpy change as a weather forecast. Just as a forecast can predict whether it will rain or shine, the enthalpy change can indicate whether a reaction is likely to proceed. A negative enthalpy change (exothermic) suggests favorable conditions, while a positive change (endothermic) may require additional energy input.
3. Calculation of Enthalpy Change
Enthalpy change can be calculated using various methods, including:
A. Hess’s Law
- Definition: Hess’s Law states that the total enthalpy change for a reaction is the sum of the enthalpy changes for individual steps, regardless of the pathway taken.
- Illustrative Explanation: Imagine taking a road trip. Whether you take a direct route or make several stops along the way, the total distance traveled (enthalpy change) remains the same. Similarly, Hess’s Law allows chemists to calculate the overall enthalpy change by adding the changes from each step of a reaction.
- Example: If a reaction can be broken down into two steps with known enthalpy changes (
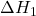 and
and 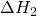 ), the total enthalpy change is:
), the total enthalpy change is:
![]()
B. Standard Enthalpy of Formation
- Definition: The standard enthalpy of formation (
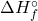 ) is the change in enthalpy when one mole of a compound is formed from its elements in their standard states.
) is the change in enthalpy when one mole of a compound is formed from its elements in their standard states. - Illustrative Explanation: Think of the standard enthalpy of formation as the recipe for baking a cake. Just as you need specific ingredients (elements) to create a cake (compound), the standard enthalpy of formation measures the energy change associated with forming a compound from its basic building blocks.
- Calculation: The enthalpy change for a reaction can be calculated using the standard enthalpies of formation of the products and reactants:
![]()
4. Types of Enthalpy Change
Enthalpy change can be categorized into several types based on the processes involved:
A. Enthalpy of Reaction
- Definition: The enthalpy change associated with a specific chemical reaction at constant pressure.
- Illustrative Explanation: Imagine a chef preparing a dish. The enthalpy of reaction is like the total energy (heat) required to cook the dish. Some recipes may require more energy (endothermic), while others may release energy (exothermic) as they cook.
B. Enthalpy of Combustion
- Definition: The enthalpy change when one mole of a substance is completely burned in oxygen.
- Illustrative Explanation: Think of the enthalpy of combustion as a campfire. When you burn wood (the substance), it releases heat and light (energy). The enthalpy of combustion measures how much energy is released during this process.
C. Enthalpy of Fusion
- Definition: The enthalpy change required to convert a solid into a liquid at its melting point.
- Illustrative Explanation: Imagine an ice cube melting in a warm room. The enthalpy of fusion is the energy needed to break the bonds holding the ice (solid) together, allowing it to become water (liquid). This process absorbs heat from the surroundings.
D. Enthalpy of Vaporization
- Definition: The enthalpy change required to convert a liquid into a gas at its boiling point.
- Illustrative Explanation: Think of boiling water on a stove. The enthalpy of vaporization is the energy needed to turn the water (liquid) into steam (gas). This process also absorbs heat, which is why steam can be hot and energetic.
5. Applications of Enthalpy Change
Enthalpy change has numerous applications across various fields:
A. Chemical Engineering
- Definition: Enthalpy change is used to design and optimize chemical processes, ensuring that reactions occur efficiently and safely.
- Illustrative Explanation: Imagine a chemical engineer as a conductor of an orchestra. Just as a conductor ensures that all musicians play in harmony, engineers use enthalpy change to balance energy inputs and outputs in chemical reactions, creating a well-orchestrated process.
B. Environmental Science
- Definition: Understanding enthalpy change helps assess the energy efficiency and environmental impact of various processes, such as combustion and energy production.
- Illustrative Explanation: Think of enthalpy change as a scale measuring the environmental impact of different fuels. Just as a scale can show the weight of an object, enthalpy change can indicate how much energy is released or consumed, helping to evaluate the sustainability of energy sources.
C. Thermodynamics
- Definition: Enthalpy change is a key concept in thermodynamics, helping to understand energy transfer and transformations in physical and chemical processes.
- Illustrative Explanation: Imagine thermodynamics as a game of energy chess. Each move (reaction) involves energy changes, and understanding enthalpy change helps players (scientists) strategize and predict the outcomes of their moves.
6. Conclusion
In conclusion, enthalpy change is a vital concept in thermodynamics that provides insight into the energy dynamics of chemical reactions and physical processes. By understanding its definition, significance, calculation methods, types, and applications, we can appreciate the role of enthalpy change in various scientific and practical contexts. Through illustrative explanations, we can visualize how enthalpy change operates in different scenarios, reinforcing the idea that it is not just a theoretical concept but a crucial parameter that influences the behavior of matter and energy in our world. As research continues to advance our understanding of thermodynamics, the applications of enthalpy change will likely expand, leading to innovative solutions in energy production, environmental sustainability, and chemical engineering.