Gay-Lussac’s Law is a fundamental principle in thermodynamics and gas behavior that describes the relationship between the pressure and temperature of a gas when the volume is held constant. Named after the French chemist Joseph Louis Gay-Lussac, this law is crucial for understanding how gases behave under varying conditions of temperature and pressure. This article will provide a detailed examination of Gay-Lussac’s Law, including its definition, mathematical expression, experimental verification, applications, and illustrative explanations to enhance comprehension.
1. Overview of Gay-Lussac’s Law
Definition: Gay-Lussac’s Law states that the pressure of a fixed amount of gas is directly proportional to its absolute temperature (measured in Kelvin) when the volume is held constant. In simpler terms, if the temperature of a gas increases, its pressure also increases, provided the volume does not change.
Illustrative Explanation: Imagine a sealed balloon filled with air. If you heat the balloon, the air inside expands, causing the pressure to increase. This is similar to how Gay-Lussac’s Law operates: as the temperature rises, the gas molecules move faster and collide with the walls of the container more forcefully, resulting in increased pressure.
2. Mathematical Expression
The mathematical expression of Gay-Lussac’s Law can be represented as:
![]()
Where:
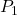 = initial pressure of the gas
= initial pressure of the gas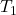 = initial absolute temperature of the gas (in Kelvin)
= initial absolute temperature of the gas (in Kelvin)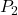 = final pressure of the gas
= final pressure of the gas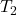 = final absolute temperature of the gas
= final absolute temperature of the gas
Illustrative Explanation: Think of this equation as a balance scale. On one side, you have the initial pressure and temperature, and on the other side, you have the final pressure and temperature. If you change the temperature, the pressure must adjust to maintain balance, illustrating the direct relationship between the two.
3. Experimental Verification
Gay-Lussac’s Law can be experimentally verified using a simple setup involving a gas-filled container, a pressure gauge, and a heat source. Here’s how the experiment typically works:
1. Setup: A gas is placed in a rigid container (such as a metal can) equipped with a pressure gauge. The container is then heated.
2. Observation: As the temperature of the gas increases, the pressure gauge shows an increase in pressure.
3. Data Collection: By recording the pressure at various temperatures, a direct relationship can be established, confirming Gay-Lussac’s Law.
Illustrative Explanation: Imagine a scientist conducting an experiment in a lab. As they heat the container, they observe the pressure gauge rising, much like watching a thermometer climb as the temperature increases. This visual representation reinforces the concept that pressure and temperature are linked.
4. Applications of Gay-Lussac’s Law
Gay-Lussac’s Law has several practical applications in various fields, including:
A. Weather Balloons
- Definition: Weather balloons are used to collect data about atmospheric pressure and temperature at different altitudes.
- Illustrative Explanation: Picture a weather balloon ascending into the atmosphere. As it rises, the temperature drops, and according to Gay-Lussac’s Law, the pressure inside the balloon decreases. This relationship helps meteorologists predict weather patterns.
B. Pressure Cookers
- Definition: Pressure cookers utilize the principles of gas behavior to cook food faster by increasing the pressure inside the sealed container.
- Illustrative Explanation: Think of a pressure cooker as a high-pressure environment for cooking. As the temperature rises, the pressure increases, allowing food to cook more quickly. This is a practical application of Gay-Lussac’s Law in everyday life.
C. Gas Storage and Transportation
- Definition: Understanding the relationship between pressure and temperature is crucial for safely storing and transporting gases, such as natural gas or propane.
- Illustrative Explanation: Imagine a gas cylinder being transported on a hot day. If the temperature inside the cylinder rises, the pressure will also increase according to Gay-Lussac’s Law. This knowledge is essential for ensuring safety and preventing accidents.
5. Limitations of Gay-Lussac’s Law
While Gay-Lussac’s Law is a valuable principle, it has limitations:
A. Ideal Gas Assumption
- Definition: Gay-Lussac’s Law assumes that the gas behaves ideally, meaning it follows the ideal gas law perfectly without any intermolecular forces or volume occupied by gas particles.
- Illustrative Explanation: Think of ideal gases as perfect spheres that never interact with each other. In reality, gases can deviate from this behavior, especially at high pressures and low temperatures, where intermolecular forces become significant.
B. Constant Volume Requirement
- Definition: The law applies only when the volume of the gas is held constant. If the volume changes, the relationship between pressure and temperature may not hold.
- Illustrative Explanation: Imagine a balloon that can expand. If you heat it, the pressure may not increase as expected because the volume is changing. This highlights the importance of maintaining constant volume for Gay-Lussac’s Law to be applicable.
6. Conclusion
In conclusion, Gay-Lussac’s Law is a fundamental principle in thermodynamics that describes the direct relationship between the pressure and absolute temperature of a gas at constant volume. By understanding its mathematical expression, experimental verification, and practical applications, we can appreciate its significance in both scientific research and everyday life. Through illustrative explanations, we can visualize how changes in temperature affect gas pressure, reinforcing the concept that these two variables are intricately linked. As we continue to explore the behavior of gases, Gay-Lussac’s Law remains a cornerstone of our understanding of thermodynamic principles, providing valuable insights into the nature of matter and its interactions. Whether in the laboratory, the kitchen, or the atmosphere, the implications of Gay-Lussac’s Law are far-reaching, making it an essential concept in the study of chemistry and physics.