Hückel’s Rule is a fundamental principle in organic chemistry that provides a criterion for determining the aromaticity of cyclic compounds. Aromatic compounds are characterized by their stability, unique electronic properties, and distinct reactivity patterns, which arise from their specific molecular structures. Hückel’s Rule states that a planar, cyclic, and fully conjugated molecule is aromatic if it contains ![]() π (pi) electrons, where
π (pi) electrons, where ![]() is a non-negative integer (0, 1, 2, …). This article will delve into the details of Hückel’s Rule, its significance, applications, and illustrative explanations to enhance understanding.
is a non-negative integer (0, 1, 2, …). This article will delve into the details of Hückel’s Rule, its significance, applications, and illustrative explanations to enhance understanding.
Definition of Hückel’s Rule
Hückel’s Rule can be succinctly stated as follows:
- A cyclic, planar, and fully conjugated molecule is aromatic if it contains
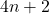 π electrons.
π electrons.
Illustrative Explanation: Think of Hückel’s Rule as a recipe for baking a cake. Just as a specific combination of ingredients is required to create a delicious cake, certain conditions must be met for a molecule to be classified as aromatic. The ![]() π electrons are like the key ingredients that ensure the cake (the molecule) turns out perfectly.
π electrons are like the key ingredients that ensure the cake (the molecule) turns out perfectly.
Key Components of Hückel’s Rule
1. Cyclic Structure: The molecule must be cyclic, meaning it forms a closed loop. This cyclic nature allows for the delocalization of π electrons around the ring.
Illustrative Explanation: Imagine a bicycle wheel. Just as the wheel is a closed loop that allows the bike to move smoothly, a cyclic structure enables the delocalization of electrons, contributing to the stability of the molecule.
2. Planarity: The molecule must be planar, meaning all atoms in the ring lie in the same plane. This planarity is essential for effective overlap of p-orbitals, which allows for the delocalization of π electrons.
Illustrative Explanation: Think of a flat piece of paper. Just as a flat surface allows for clear visibility of all points on the paper, planarity in a molecule ensures that the p-orbitals can overlap effectively, facilitating electron delocalization.
3. Fully Conjugated System: The molecule must have a fully conjugated system, meaning that there are alternating single and double bonds (or lone pairs) around the ring. This conjugation allows for the overlap of p-orbitals, enabling the delocalization of π electrons.
Illustrative Explanation: Imagine a chain of people holding hands, where each person represents an atom. Just as the chain can only form if everyone is connected, a fully conjugated system requires that all atoms in the ring are connected through alternating bonds, allowing for the flow of electrons.
4. ![]() π Electrons: The final criterion is that the molecule must contain
π Electrons: The final criterion is that the molecule must contain ![]() π electrons, where
π electrons, where ![]() is a non-negative integer. This specific number of π electrons is what imparts aromatic stability to the molecule.
is a non-negative integer. This specific number of π electrons is what imparts aromatic stability to the molecule.
Illustrative Explanation: Think of the ![]() π electrons as the perfect number of candles on a birthday cake. Just as having the right number of candles makes the cake celebration special, having the right number of π electrons gives the molecule its aromatic character.
π electrons as the perfect number of candles on a birthday cake. Just as having the right number of candles makes the cake celebration special, having the right number of π electrons gives the molecule its aromatic character.
Examples of Hückel’s Rule
1. Benzene: Benzene (![]() ) is the classic example of an aromatic compound. It has a cyclic structure, is planar, has alternating single and double bonds, and contains 6 π electrons (where
) is the classic example of an aromatic compound. It has a cyclic structure, is planar, has alternating single and double bonds, and contains 6 π electrons (where ![]() in
in ![]() ).
).
![]()
Illustrative Explanation: Imagine benzene as a perfectly balanced seesaw. Just as the seesaw remains stable when perfectly balanced, benzene remains stable due to its ![]() π electrons, allowing for delocalization and aromaticity.
π electrons, allowing for delocalization and aromaticity.
2. Naphthalene: Naphthalene (![]() ) consists of two fused benzene rings. It is cyclic, planar, fully conjugated, and contains 10 π electrons (where
) consists of two fused benzene rings. It is cyclic, planar, fully conjugated, and contains 10 π electrons (where ![]() ).
).
![]()
Illustrative Explanation: Think of naphthalene as a double-decker bus. Just as the bus has two levels that are connected and stable, naphthalene has two interconnected benzene rings that maintain aromatic stability through their ![]() π electrons.
π electrons.
3. Cyclobutadiene: Cyclobutadiene (![]() ) is a cyclic compound with alternating double bonds. However, it contains only 4 π electrons (where
) is a cyclic compound with alternating double bonds. However, it contains only 4 π electrons (where ![]() ), which does not satisfy Hückel’s Rule.
), which does not satisfy Hückel’s Rule.
![]()
Illustrative Explanation: Imagine cyclobutadiene as a wobbly table with only four legs. Just as a table with fewer legs than needed is unstable, cyclobutadiene is unstable due to its insufficient number of π electrons, making it non-aromatic.
Applications of Hückel’s Rule
1. Predicting Aromaticity: Hückel’s Rule is widely used to predict whether a given cyclic compound is aromatic, non-aromatic, or anti-aromatic. This prediction is crucial for understanding the stability and reactivity of organic compounds.
Illustrative Explanation: Think of Hückel’s Rule as a set of traffic lights for chemists. Just as traffic lights guide drivers on when to stop or go, Hückel’s Rule helps chemists determine whether a compound is aromatic (go) or not (stop).
2. Designing New Compounds: Chemists use Hückel’s Rule to design new aromatic compounds for various applications, including pharmaceuticals, materials science, and organic electronics. Understanding aromaticity allows for the development of compounds with desired properties.
Illustrative Explanation: Imagine a chef creating a new dish. Just as a chef uses specific ingredients to achieve a particular flavor, chemists use Hückel’s Rule to select the right molecular structure to create compounds with specific characteristics.
3. Understanding Reaction Mechanisms: The concept of aromaticity is essential in understanding the mechanisms of many organic reactions, such as electrophilic aromatic substitution. Aromatic compounds often undergo reactions that preserve their aromatic character.
Illustrative Explanation: Think of aromatic compounds as skilled dancers in a performance. Just as dancers must maintain their rhythm and form while moving, aromatic compounds must preserve their aromaticity while undergoing chemical reactions.
Limitations of Hückel’s Rule
While Hückel’s Rule is a powerful tool for predicting aromaticity, it has limitations:
1. Non-Planar Structures: Hückel’s Rule does not apply to non-planar cyclic compounds, even if they contain the correct number of π electrons. Planarity is essential for effective p-orbital overlap.
Illustrative Explanation: Imagine a jigsaw puzzle piece that is bent. Just as a bent piece cannot fit into the puzzle, a non-planar compound cannot achieve aromaticity, regardless of its π electron count.
2. Complex Systems: In larger and more complex systems, other factors such as steric hindrance and electronic effects may influence aromaticity, making it necessary to consider additional criteria beyond Hückel’s Rule.
Illustrative Explanation: Think of a complex orchestra with many instruments. Just as the harmony of the music depends on more than just the notes played, the aromaticity of complex compounds depends on various factors beyond just the number of π electrons.
Conclusion
Hückel’s Rule is a cornerstone of organic chemistry that provides a clear criterion for determining the aromaticity of cyclic compounds. By understanding the key components of Hückel’s Rule—cyclic structure, planarity, full conjugation, and the ![]() π electrons—chemists can predict the stability and reactivity of various organic molecules. The applications of Hückel’s Rule extend to predicting aromaticity, designing new compounds, and understanding reaction mechanisms, making it an invaluable tool in the field of chemistry. As research continues to explore the complexities of aromatic compounds, Hückel’s Rule will remain a fundamental principle guiding our understanding of molecular behavior and interactions.
π electrons—chemists can predict the stability and reactivity of various organic molecules. The applications of Hückel’s Rule extend to predicting aromaticity, designing new compounds, and understanding reaction mechanisms, making it an invaluable tool in the field of chemistry. As research continues to explore the complexities of aromatic compounds, Hückel’s Rule will remain a fundamental principle guiding our understanding of molecular behavior and interactions.