Interhalogen compounds are fascinating chemical species formed from the combination of two or more different halogen elements. These compounds exhibit unique properties and reactivities that distinguish them from both the individual halogens and other types of chemical compounds. This article will delve into the definition, types, properties, synthesis, applications, and significance of interhalogen compounds, accompanied by illustrative explanations to enhance understanding.
1. Definition of Interhalogen Compounds
Definition: Interhalogen compounds are chemical compounds that consist of two or more different halogen atoms. The halogens are the elements found in Group 17 of the periodic table, which include fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At). Interhalogen compounds can be represented by the general formula ![]() , where
, where ![]() and
and ![]() are different halogens, and
are different halogens, and ![]() indicates the number of halogen atoms of type
indicates the number of halogen atoms of type ![]() bonded to
bonded to ![]() .
.
Illustrative Explanation: Imagine a diverse group of friends (halogens) coming together to form a unique club (interhalogen compound). Each friend brings their own personality (chemical properties) to the club, creating a new dynamic that is different from any individual member. For example, if chlorine (Cl) and fluorine (F) form a club, they create a new entity (interhalogen compound) with distinct characteristics that reflect the combination of their traits.
2. Types of Interhalogen Compounds
Interhalogen compounds can be classified based on their composition and structure:
A. Binary Interhalogen Compounds
- Definition: These compounds consist of two different halogen atoms. They can be represented by the general formula
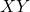 , where
, where 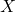 and
and 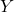 are different halogens.
are different halogens. - Examples:
– Chlorine trifluoride (ClF₃): A compound formed from chlorine and fluorine.
– Bromine monochloride (BrCl): A compound formed from bromine and chlorine.
Illustrative Explanation: Think of binary interhalogen compounds as pairs of dancers (halogens) performing a duet. Each dancer brings their own style and flair to the performance, resulting in a captivating routine (the unique properties of the compound) that showcases the strengths of both dancers.
B. Ternary Interhalogen Compounds
- Definition: These compounds consist of three different halogen atoms and can be represented by the general formula
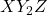 or
or 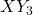 .
. - Examples:
– Bromine trifluoride (BrF₃): A compound formed from bromine and fluorine.
– Iodine pentafluoride (IF₅): A compound formed from iodine and fluorine.
Illustrative Explanation: Imagine ternary interhalogen compounds as a trio of musicians (three different halogens) forming a band. Each musician contributes their unique instrument (chemical properties), and together they create a harmonious piece of music (the compound) that is richer and more complex than any solo performance.
3. Properties of Interhalogen Compounds
Interhalogen compounds exhibit several distinctive properties that arise from their unique compositions:
A. Physical Properties
- State of Matter: Many interhalogen compounds are gases or liquids at room temperature, with some being solids. For example, chlorine trifluoride (ClF₃) is a colorless gas, while iodine monochloride (ICl) is a solid.
- Color: Interhalogen compounds can exhibit a range of colors. For instance, bromine monochloride (BrCl) is a yellowish-brown liquid, while iodine monochloride (ICl) appears as a reddish-brown solid.
Illustrative Explanation: Think of interhalogen compounds as a box of crayons, where each crayon (compound) has its own color (physical properties). Just as the colors can vary widely, so too can the states and appearances of these compounds, reflecting the diversity of their halogen components.
B. Chemical Properties
- Reactivity: Interhalogen compounds are generally more reactive than the individual halogens. This increased reactivity is due to the presence of different halogen atoms, which can lead to unique reaction pathways.
- Formation of Halides: Interhalogen compounds can react with metals to form metal halides. For example, when chlorine trifluoride (ClF₃) reacts with sodium (Na), it forms sodium fluoride (NaF) and sodium chloride (NaCl).
Illustrative Explanation: Imagine interhalogen compounds as adventurous explorers (reactive compounds) eager to discover new territories (react with other substances). Their diverse backgrounds (different halogens) give them the courage to venture into new areas, leading to exciting discoveries (new compounds).
4. Synthesis of Interhalogen Compounds
Interhalogen compounds can be synthesized through various methods, including:
A. Direct Combination
- Definition: Interhalogen compounds can be formed by the direct reaction of two different halogens under controlled conditions.
- Example: Chlorine and fluorine can react to form chlorine trifluoride (ClF₃) when mixed in a controlled environment.
Illustrative Explanation: Think of direct combination as a cooking recipe where two ingredients (halogens) are mixed together in a pot (reaction vessel) to create a new dish (interhalogen compound). The right conditions (temperature, pressure) ensure that the flavors blend perfectly, resulting in a delicious meal.
B. Halogen Exchange Reactions
- Definition: Interhalogen compounds can also be synthesized through halogen exchange reactions, where one halogen in an existing interhalogen compound is replaced by another halogen.
- Example: Bromine trifluoride (BrF₃) can react with iodine to form iodine trifluoride (IF₃) through a halogen exchange reaction.
Illustrative Explanation: Imagine a game of musical chairs where players (halogens) switch seats (positions) with each other. In this game, one player (the halogen being replaced) leaves their seat, and a new player (the incoming halogen) takes their place, resulting in a new arrangement (interhalogen compound).
5. Applications of Interhalogen Compounds
Interhalogen compounds have several important applications in various fields:
A. Chemical Synthesis
- Definition: Interhalogen compounds are often used as reagents in organic synthesis, facilitating the introduction of halogen atoms into organic molecules.
- Example: Chlorine trifluoride (ClF₃) is used in the synthesis of fluorinated organic compounds, which have applications in pharmaceuticals and agrochemicals.
Illustrative Explanation: Think of interhalogen compounds as specialized tools in a chemist’s toolbox. Just as a specific tool can help a craftsman create intricate designs, interhalogen compounds enable chemists to build complex organic structures with precision.
B. Oxidizing Agents
- Definition: Many interhalogen compounds serve as powerful oxidizing agents in chemical reactions, allowing for the oxidation of various substrates.
- Example: Bromine trifluoride (BrF₃) is used as an oxidizing agent in the production of fluorinated compounds and in certain analytical chemistry applications.
Illustrative Explanation: Imagine interhalogen compounds as superheroes with special powers (oxidizing abilities). Just as superheroes can help others achieve their goals (oxidation of substrates), interhalogen compounds facilitate important chemical transformations.
6. Conclusion
In conclusion, interhalogen compounds are a unique and diverse class of chemical species formed from different halogen elements. Their distinctive properties, reactivities, and applications make them significant in both organic synthesis and industrial processes. Understanding interhalogen compounds enhances our knowledge of chemical bonding, reactivity, and the intricate relationships between different elements in the periodic table. As we continue to explore the chemistry of interhalogen compounds, we can appreciate their role in advancing scientific research and developing new materials and technologies. Whether as tools in a chemist’s lab or as components in industrial processes, interhalogen compounds are essential players in the world of chemistry.